INTRODUCTION
Birds play important roles in ecosystems, such as in the control of plagues, pollination, and seed dispersal, and are therefore a taxonomic group of critical importance in natural restoration processes for forest conservation (SODHI et al., 2011). The ecological functions of birds are even more relevant now that habitat transformations due to human activities, such as forestry, mining, and agriculture, are some of the main threats to the biological diversity of the planet (JANTZ et al., 2003; SODHI et al., 2011; PATTEN & SMITH-PATTEN, 2012).
One of the most threatened ecosystems in the tropics is dry forest (TDF), due to soil fertility issues (PORTILLO-QUINTERO et al., 2013; BANIN et al., 2014; CAO et al., 2015; TRILLERAS et al., 2015). Great swaths of this ecosystem have been transformed into cultivation areas and human settlements (JANZEN, 1988; ARCILA et al., 2012; MARINARO et al., 2014; GASPARRI et al., 2015). The valley of the upper Magdalena River, on the western and eastern foothills of the Eastern and Central cordilleras of the Colombian Andes, consists of tropical dry forest (HOLDRIDGE, 1967). This is an area of lowlands with notable ecosystem mosaics (ACOSTA-GALVIS et al., 2006).
The geographic valley of the Magdalena River, along with the tropical dry forests of the Caribbean region of Colombia, are considered focal areas of endemism for birds associated with the Neotropical dry forest (CRACRAFT, 1985; STOTZ et al., 1996; STATTERSFIELD et al., 1998; MYERS et al., 2000). Information on bird species of the tropical dry forests of Colombia has been based mainly on inventories and lists of birds from specific regions or locations (NARANJO, 1992; STILES et al., 1995; ESTELA & LÓPEZ-VICTORIA, 2005; LOSADA-PRADO et al., 2005. There are few studies that describe the ecology of the avifauna and species dominance, or specific analyses of the bird community of this ecosystem (e.g. LOSADA-PRADO & MOLINA-MARTÍNEZ, 2012).
The composition and structure of bird communities is influenced by historical processes that have marked their geographical distribution (GASTON & BLACKBURN, 2000), as well as by recent ecological processes such as human disturbance (SODHI et al., 2011). The latter factor influences habitat preferences, behavior, and the reproductive success of species. However, response differs depending on the level of transformation and on the structure and composition of remaining habitats (DURÁN & KATTAN, 2005). When the levels of alteration are elevated, the remaining forest cover does not differ in structure and composition from the human matrix (RENGIFO, 1999).
The present study was carried out to evaluate and compare the composition, diversity, and trophic structure of the bird assemblage in two landscape units of a tropical dry forest located in the jurisdiction of Guarinocito, La Victoria, Caldas, Colombia. The objective was to identify whether the habitat modifications caused by human intervention (mining and livestock farming) have caused a decrease in the structure and composition of the bird community associated with the remaining forest cover.
MATERIALS AND METHODS
Three sampling periods of five days each, were carried out between April 2013 and October 2014. Sampling occurred in the “Hacienda La Española” (5° 21’ 51.9’’ N, 74° 47’ 45.7’’ W), jurisdiction of Guarinocito, municipality of La Victoria, Caldas, Colombia (Figure 1). The area corresponds to tropical dry forest, typical of the Magdalena Valley (DEVIA et al., 2003). The altitude above sea level ranges between 240 and 280 m. There is a bimodal precipitation regime, with four seasons corresponding to two rainy seasons (May-June and September-November) and two dry seasons (January-April and July-August). The average annual precipitation is 1590 mm and the average temperature is 28.2°C (CORPOCALDAS, 2001).

Figure 1 Geographical localization of Hacienda La Española in Caldas department. (Source: Google Earth Pro ®).
Two contrasting landscape units were identified in the study area. One intervened area comprised 75 ha destined to livestock farming) with a vegetation dominated by shrubs and grasses, wetlands (swamps and oxbow lakes), and mining activities (stone extraction and artisanal gold extraction), and a 90 ha of forested area corresponding to dry forest fragments that are in natural regeneration.
The avifauna present in each landscape unit was evaluated using point counts and mist netting. The sampling effort was equal in each unit, with the same number of point counts and mist nets. Sampling was carried out twice per day, between 06:00 and 10:00, and between 15:30 and 18:30. Each point count consisted of an observer using binoculars and recording birds seen within a previously established 50 m radius during 10 min. Point count stations were located 100 to 200 m apart (RALPH et al., 1996). The species, number of individuals, and type of substrate on which the observed specimen was at the time of observation were recorded. Mist nets were 12 m long by 3 m high with 16 mm mesh size, and were used as a complementary method to increase the probability of recording fast-flying species. Three double nets were located randomly within the study area each sampling day, covering a total of 72 m2. Nets were checked every hour from 07:00 until 18:00 hours. Specimen identification was carried out in the field following the taxonomic guides of HILTY & BROWN (1986) and considering the geographic distribution described by the IUCN (2014).
A sampling unit was defined as all bird records obtained during two points counts as well as records from mist nets, establishing in this way 30 sampling units for each landscape unit during the entire study period. Species accumulation curves were constructed using this information. Sample representativeness was determined based on the species richness estimators Chao2, Bootstrap, and first- and second-order Jackknife (MORENO, 2001), which were calculated using the program Estimates® v8.2.0. (COLWELL, 2012). The Shannon entropy was calculated as 1D=exp(H’) (JOST, 2006) to obtain the true diversity value, and Simpson’s dominance index (Ds) was calculated as D_s=1-λ (BROWER & ZAR, 1984) for each landscape unit. The diversity between landscape units was compared with a t test modified by Hutcheson (tH) (HUTCHESON, 1970; MAGURRAN, 2004), and dominance was compared using a computational resampling technique “Bootstrap” (KREBS, 1999). Rarefaction curves were also constructed to compare species richness between landscape units. Abundance data was transformed using the function log10(x+1) to reduce the effect of very abundant species, and the degree of similarity in assemblage structure was compared using the Bray-Curtis index calculated from the transformed abundance data. These analyses were carried out using the software PAST® version 2.17 (HAMMER et al., 2001).
The trophic structure analysis was performed by classifying each species according to its diet. A total of 11 trophic guilds were identified as follows. F: frugivores, I: insectivores, N: nectarivores, S: seed-eaters, C: carnivores, FI: frugivores and insectivores, FS: frugivores and seed-eaters, NI: nectarivorous and insectivores, CI: carnivores and insectivores, FNI: frugivores, nectarivores, and insectivores, FCI: frugivores, carnivores, and insectivores, and SIF: seed-eaters, insectivores, and frugivores. The omnivorous guild was ignored as it does not provide a clear representation of resource use (RIVERAGUTIÉRREZ, 2006). Although trophic guild classification was performed following guidelines by PEARSON (1975) and STILES & ROSSELLI (1998), food capture techniques or foraging height were not considered. The proportion of each trophic guild within each landscape unit was calculated, and landscape units were compared by performing a contingency table analysis using a Pearson’s X 2 test (PLACKETT, 1983).
RESULTS
A total of 122 points counts were performed and 4,392 m of mist nets were installed. This sampling effort resulted in 1,005 records, corresponding to 127 species distributed in 18 orders and 42 families. The most abundant species were Thraupis episcopus (7.5%), Sicalis flaveola (6.8%), Forpus conspicillatus (5.4%), Pitangus sulphuratus (3.6%), Columbina talpacoti (3.4%), Egretta thula (3.1%), and Tyrannus melancholicus (2.5%).
There was a total of 722 birds detected in the intervened area, belonging to 94 species, 16 orders, and 33 families. The following species had the highest number of records in this landscape unit: S. flaveola (9.4%), T. episcopus (8.2%), F. conspicillatus (6.78%), C. talpacoti (4.6%), and E. thula (4.3%). There was a total of 283 birds detected in the forested area, belonging to 79 species, 15 orders, and 31 families. The following species had the highest number of records in this landscape unit: Myiothlypis fulvicauda (17.6%), Coereba flaveola (5.6%), T. episcopus (5.3%), Catharus ustulatus (4.6%), and Troglodytes aedon (4.2%) (Table 1).
Of the 127 species recorded in the study area, 49 species were recorded only in the intervened landscape unit, of which six species were migratory (Himantopus mexicanus, Hirundo rustica, Machetornis rixosa, Porphyrio martinicus, and Tringa flavipes), and one species was endemic (Myiarchus apicalis). On the other hand, 34 species were recorded only in the forested landscape unit, of which only Parkesia noveboracensis was migratory. One migratory species was recorded in both the intervened and forested areas (Actitis macularia), and a vulnerable endemic species (Ortalis columbiana) was recorded in both landscape units (Table 1).
Table 1 Birds species observed in Hacienda La Española, La Victoria, Caldas. IN: intervened zone. BO: dry forest zone. IN-BO: intervened and dry forest zone. TG: trophic guild, EN-MI: endemic-migratory. IUCN: global threats (IUCN, 2014).

Between 68.2% and 86.0% of potential bird species from the Hacienda La Española were recorded during sampling. Between 65.7% and 85.4% of potential species were recorded in the intervened landscape unit, whereas between 58.9 and 82.8% of potential species were recorded in the forested landscape unit (Table 2). There were no significant differences between the two landscape units in the degree of uncertainty associated with the identity of the species to which a randomly selected individual was assigned (THutchinson=1.59, p=0.81). However, species richness was lower in the forested area (79 species) than in the intervened area (95 species), and dominance was slightly higher in the intervened area (p value Ds bootstrap = 0.029). True diversity was 50.9 effective species in the forested area, whereas true diversity was 45.6 effective species in the intervened landscape unit. The true diversity estimate for the entire study area was 62.2 effective species (Table 3), with a degree of similarity between the two units in assemblage composition of 43.47%.
Table 2 Efficiency of sampling effort in Hacienda La Española (HE) and each landscape unit.

BO: dry forest. IN: intervened zone.
Table 3 Ecological attributes of the bird assemble in Hacienda La Española (HE) and each landscape unit.
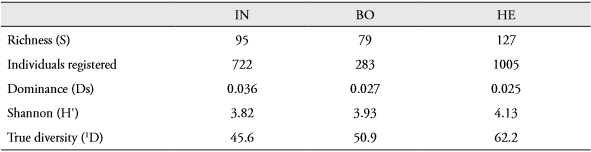
BO: dry forest. IN: intervened zone.
The most representative trophic guilds were the insectivores (I) with 20.5% of species in the forested unit and 29.4% of species in the intervened unit, followed by frugivores and insectivores (F, I), with 15.1% of species in the forested unit and 19.6% of species in the intervened unit (Figure 2). Cathartes aura and Coragyps atratus were recorded in the intervened unit; these were the only two species classified within the scavenger trophic guild (S). There were no significant differences in trophic structure between the two landscape units (X 2 =14.60, p=0.33).
DISCUSSION
Despite being mobile organisms, birds are sensitive to changes in habitat structure (CLEARY et al., 2005; DÍAZ et al., 2005). For this reason, they constitute excellent models to understand the response of wild animal communities to environmental modifications, especially those generated by human activities (MACGREGOR-FORS & SCHONDUBE, 2011). The ability of birds to move through landscapes probably results in the persistence of populations in fragmented habitats such as those found in the study area, and can modulate the structure and composition of bird assemblages at a given location (GILLIES & CLAIRE, 2010).
The dry forest fragments found in the Hacienda la Española are small and far from each other (VARGAS-FIGUEROA et al., 2016), and birds probably move between fragments looking for the food resources they need (e.g. MERRIAM, 1984; BAUDRY & MERRIAM, 1988; OPDAM, 1990; TAYLOR et al., 1993; BEIER & NOSS, 1998; RENGIFO, 1999; FAHRIG, 2003; URIARTE et al., 2011). Due to the size and shape of fragments, there could be an enhanced edge effect, which facilitates the occurrence of generalist species in the forest, and would result in bird assemblages with similar diversity attributes (BENETTE & SAUNDERS, 2010; MORTELLITI et al., 2010; PINO et al., 2010; SMITH et al., 2011).
Other elements that can modulate local diversity of bird species in a fragmented habitat such as the dry forest of the Hacienda la Española are microclimatic conditions and habitat structural complexity. According to COLLINGE (1996), when habitats become fragmented the microclimatic conditions are altered drastically, affecting significantly the plant and animal communities. These microclimatic conditions are generally defined by the vegetation structure and local topography (SUGGITT et al., 2011). In thermal conditions, such as those found in the study area, the expected microclimatic differences between forested and intervened areas can be a determinant factor for bird diversity, as suggested by PATTENT et al. (2012) in a study carried out in lowland forests of Belize and Costa Rica.
Habitat structural complexity tends to decrease in fragmented forests due to the use of tree species from the understory by local residents, which leads to a simplification of the habitat that affects local species diversity (TEWS et al., 2004). Plant species typical of open areas were observed inside the forest fragments of the study area, which suggests that these fragments are a mix of the original forest and secondary growth (RENGIFO, 1999; DURÁN & KATTAN, 2005), where habitat structure has been simplified.
It has been widely demonstrated that the establishment of livestock farming and/or mining exploitation within dry forest matrices has a negative effect on the abundance and structure of bird assemblages (NARANJO, 1992; SAAD & PETIT, 1992; ESTRADA & COATES-ESTRADA, 2005; TSCHARNTKE et al., 2008). Areas with livestock farming and mining exploitation are inadequate habitats for forest species, due to their low structural complexity and low availability of food resources (MARTIN, 1984; ESTRADA et al., 1997; HUGHES et al., 2002; TSCHARNTKE et al., 2008). The bird assemblages that are found in landscapes under pressure from human activities usually lack species that are sensitive to perturbations, as those species are usually the first to become extinct locally due to processes caused by humans such as the transformation or degradation of the habitat (STOTZ et al., 1996; PETIT & PETIT, 2003).
The lack of difference in trophic structure between the assemblages of the two evaluated landscape units in the Hacienda La Española is probably the result of a lack of difference in food resource availability, or of the species using both landscape units to obtain resources (e.g. BLAKE & LOISELLE, 2001; HERZOG & KESSLER, 2002). The differential effect of species recorded in only one landscape unit would therefore be reduced, because the feeding preferences of species that use both landscape units would result in a decrease in the functional differences of the bird assemblages found in the two units. However, it should be noted that despite a lack of differences in diversity or trophic composition between the assemblages, the similarity percentage was low (<50%), which suggests that there are differences in structure between the assemblages.
Moreover, the species recorded in the Hacienda La Española during this study correspond to species frequently found in areas with high human perturbation, and the presence of threatened species was low. It is therefore necessary to incorporate other variables in future studies, such as the climatic season or the migratory patterns of species (SKIRVIN, 1981; PETIT & PETIT, 2003; MOORE et al., 2004; GÓMEZ et al., 2015), information that would provide a better idea of the effect of human perturbation on the dry forests of the Magdalena Valley. This kind of evidence is a key factor for the planning of conservation strategies for the tropical dry forest remnants, one of the natural elements of highest conservation interest for national as well as international scientific communities in the Neotropic region (CEBALLOS & GARCÍA, 1995; TURNER & CORLETT, 1996; SÁNCHEZ-AZOFEIFA et al., 2005; PORTILLO-QUINTERO & SÁNCHEZ-AZOFEIFA, 2010; GARCÍA et al., 2014; PRIETO-TORRES et al., 2016).















