INTRODUCTION
The white-footed tamarin (Saguinus leucopus) is a Neotropical, endemic and monotypic primate of Colombia (DEFLER, 2010). The Saguinus genus has morphological adaptations that allow for a quadrupedal locomotion mainly with jumps to suspend itself in branches and on the trunks of the trees (DEFLER, 2010; YOULATUS & MELDRUM, 2011; YOULATUS, 2018). They have claws on their hands that allow them to adhere to and to remove the bark of the trees and feed off the latex that flows from these trees (ANKEL-SIMONS, 2007; MORALES-JIMÉNEZ et al., 2008; DEFLER, 2010). The strength for this is supplied by the muscles of the appendicular skeleton, mainly by the digital flexor muscles of the forearm (KARDONG, 2012), therefore the bones should be adapted to a more specialized locomotor type (FLEAGLE, 2013) with the humerus, radius and ulna in S. leucopus developing bony reliefs that serve as the origins of these muscles and thus permitting arboreal and quadrupedal locomotion (DUQUE-PARRA & VÉLEZ-GARCÍA, 2012; DUQUE-PARRA et al., 2013; VÉLEZ-GARCÍA et al., 2016).
Along the caudomedial part of the forearm in primates there are the digital flexor muscles of the hand such as the flexor digitorum superficialis muscle and flexor digitorum profundus muscle, which are necessary to provide the strength to flex the articulations of the digits and be able to grab onto trees in quadrupedal locomotion (VÉLEZ-GARCÍA & CASTAÑEDA, 2016). The anatomic adaptations of these muscles might be a morphological response to environmental challenges such as the movement between trees for feeding, socialization and escape of potential predators (YOULATUS, 2018). Thus, the main objective of this study was to describe the gross anatomy of these muscles in terms of shape, origin, insertion, innervation and arterial supply; in order to recognize the morphological disposition in comparison with other primates. This will also give anatomical bases for clinical diagnosis, and surgical and orthopaedic approaches in the caudomedial part of the forearm (VÉLEZ-G & CASTAÑEDA, 2016), since the musculoskeletal alterations in this species are one of the most presented in wildlife care centres (VARELA et al., 2010).
METHODS
Ten dead specimens of Saguinus leucopus were used (five females and five males) with weights between 300 and 460 grams. They died of natural causes between 2012 and 2013 in wildlife care centres of CORPOCALDAS (Corporación Autónoma Regional de Caldas), the environmental authority of department of Caldas in Colombia. After necropsy, they were fixed via subcutaneous, intramuscular and intracavity infiltrations with a solution of formaldehyde 10 %, mineral oil 5 % and phenic acid 1 %. Subsequently they were submerged in the same solution but without mineral oil for a minimum of one week. Both thoracic limbs were dissected from superficial to deep, and photographic records of the procedure were made in the Veterinary Anatomy laboratories of the Universidad del Tolima and the Universidad de Caldas. We did an anatomical descriptive study where we emphasized the gross anatomy of the flexor digitorum superficialis (M. flexor digitorum superficialis) and flexor digitorum profundus muscles (M. flexor digitorum profundus), describing the findings according to the terminology of the Nomina Anatomica Veterinaria (INTERNATIONAL COMMITTEE ON VETERINARY GROSS ANATOMICAL NOMENCLATURE, 2017) and the anatomical findings reported of humerus, radio and ulna in this species (DUQUE-P & VÉLEZ-G, 2014; DUQUE-P et al., 2014; VÉLEZ-G et al., 2016).
RESULTS
Flexor digitorum superficialis muscle (M. flexor digitorum superficialis)
The flexor digitorum superficialis muscle had a tendinous origin from a common tendon with the adjacent muscles (M. palmaris longus, M. flexor carpi ulnaris and humeral heads of the M. flexor digitorum profundus) in the medial epicondyle of the humerus and in the adjacent intermuscular septa. In the middle part of the forearm the development of four muscular bellies was observed (lateral, intermediate, medial and deep) from which each one developed a tendon, where the medial belly went to the digit III, the deep belly to the digit II, the intermediate belly to the digit IV, and the lateral belly went to the digit V (Figure 1). The latter originated from the tendon and belly of the deep humeral head of the flexor digitorum profundus muscle (Figure 2). Each tendon at the level of the metacarpal-phalangeal joint bifurcated to give way to the tendons of the flexor digitorum profundus muscle, developing the flexor manica (Manica flexoria). At the level of the proximal interphalangeal joint it bifurcates to insert onto both sides of the middle part of the middle phalanx of the digits II-V. It was innervated by the median nerve and its arterial supply was performed by the median and ulnar arteries.
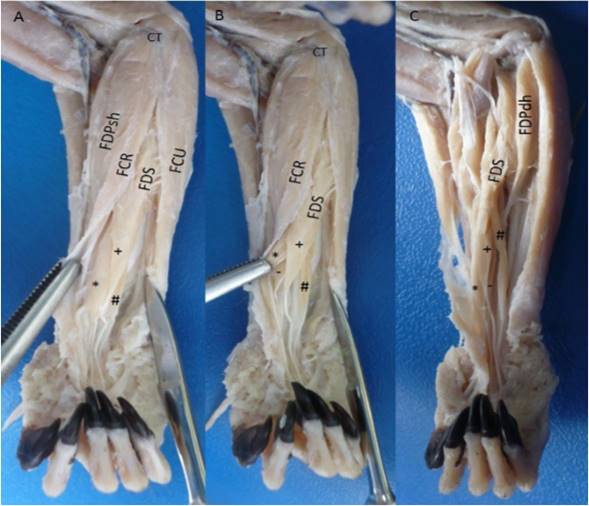
Figure 1 Flexor digitorum superficialis muscle in the right forearm of Saguinus leucopus. A-B. Superficial views; C. Deep view. FDS: Flexor digitorum superficialis with their *: Medial belly, +: Intermediate belly, #: Lateral belly and -: Deep belly; FDP: Flexor digitorum profundus, FDPsh: Superficial humeral head, FDPdh: Deep humeral head, FCR: Flexor carpi radialis, FCU: Flexor carpi ulnaris, CT: Common tendon of origin from medial epicondyle of the humerus.
Flexor digitorum profundus muscle (M. flexor digitorum profundus)
The flexor digitorum profundus muscle had six heads: Two humerals (superficial and deep), one radial, one cubital, and two ulnar (superficial and deep). The superficial humeral head had a tendinous origin in the medial epicondyle of the humerus by a common tendon with the adjacent muscles. The radial head originated in the antebrachial fascia, proximal two thirds of the caudal surface and interosseous margin of the radius; and the medial surface of the antebrachial interosseous membrane. The superficial humeral head joined with the radial head developing a bipennate humeroradial complex (Figure 2). The deep humeral head was fusiform with a tendino-muscular origin in the medial epicondyle of the humerus and the adjacent intermuscular septa with the flexor digitorum superficialis and flexor carpi ulnaris muscles. This head seemed to be part of the flexor digitorum superficialis muscle, but after carefully dissection they were found to be separate, however a belly of the later muscle was strongly originated from this head. The cubital head had a fleshy origin in the medial collateral ligament of the elbow. The deep ulnar head had a fleshy origin in the proximal two thirds of the cranial part of the medial surface, cranial surface, interosseous margin of the ulna and the lateral surface of the antebrachial interosseous membrane. The deep ulnar head joined with the cubital head and developed a bipennate cubito-ulnar complex. The superficial ulnar head was unipennate with a fleshy origin from the medial surface of the olecranon until the proximal half of the caudal part of the medial surface of the ulna.
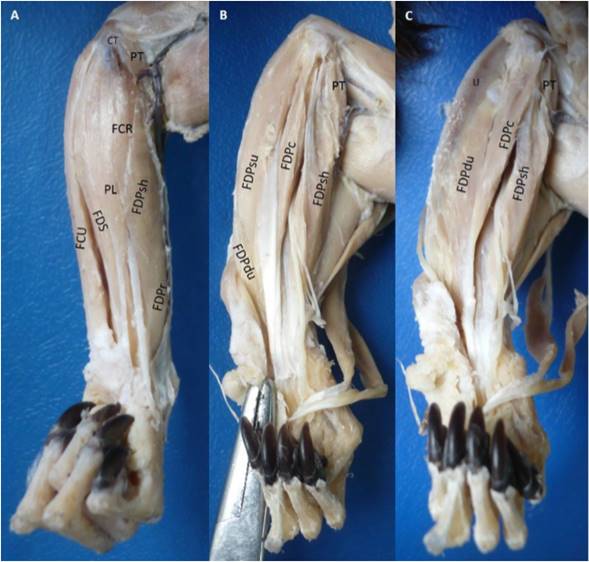
Figure 2 Flexor digitorum profundus muscle in the left forearm of Saguinus leucopus. A. Superficial view; B-C. Deep views. PT: Pronador teres, FCR: Flexor carpi radialis, PL: Palmaris longus, FDS: Flexor digitorum superficialis, CT: Common tendon of origin from medial epycondile of the humerus, U: Ulna, FCU: Flexor carpi ulnaris, FDP: Flexor digitorum profundus, FDPsh: Superficial humeral head, FDPr: Radial head, FDPc: Cubital head, FDPdu: Deep ulnar head, FDPsu: Superficial ulnar head.
All the tendons fused proximally to the carpal canal and passed through it, and distally it was divided in five tendons for each digit (Figure 2). Each tendon was highly developed with the presence of a groove that makes it look double and they were inserted onto the palmar part of the base of the distal phalanx of each digit.
The two heads of the humeroradial complex were innervated by the median nerve; the superficial ulnar and deep humeral heads by the ulnar nerve; the cubital head by the median and ulnar nerves; and the deep ulnar head by the median nerve.
The arterial supply to superficial humeral head was performed by branches of the median and ulnar arteries, and the radial head by the median, radial and caudal interosseous arteries. The cubital head was supplied by the ulnar and median arteries and the deep ulnar head by the ulnar and caudal interosseous arteries. The deep humeral head by the recurrent ulnar artery and the superficial ulnar head by the recurrent ulnar and caudal interosseous arteries.
DISCUSSION
Flexor digitorum superficialis muscle (M. flexor digitorum superficialis)
The presence of a single humeral head as in Saguinus leucopus can also be found in other primates such as Cebus albifrons (CRIBILLERO et al., 2009), Cebus olivaceus (YOULATUS, 2000), Macaca fascicularis, Macaca mulatta (KIMURA & TAKAI, 1970; ACKERMANN, 2003) and Papio hamadryas (CHAMPNEYS, 1871; ACKERMANN, 2003). In other species, two humeral heads have been reported, but both originated in the medial epicondyle of the humerus as in Alouatta seniculus, Ateles paniscus (YOULATUS, 2000) and Callimico goeldii (OSMAN-HILL, 1959). However in the later species it is reported that the tendon gives fibers to deep flexor tendon (OSMAN-HILL, 1959), which can be similar to S. leucopus, but we corroborate that the tendon had its own belly, therefore it was considered as one of the humeral heads of the flexor digitorum profundus muscle, deep humeral head.
In Homo sapiens sapiens the flexor digitorum superficialis muscle has two heads, humero-ulnar and radial (FEDERATIVE INTERNATIONAL COMMITTEE ON ANATOMICAL TERMINOLOGY, 1998; STANDRING, 2016) as in Pan troglodytes (CHAMPNEYS, 1871; DIOGO et al., 2012). In Pongo pygmaeus the ulnar head is originated in the coronoid process of the ulna and the olecranon without origin in the humerus (PRIMROSE, 1900; ACKERMANN, 2003). The absence of the radial head in Homo sapiens sapiens is considered an anatomical variant (STANDRING, 2016). In Sapajus libidinosus and Sapajus apella there are the humeral and radial heads (AVERSI-F et al., 2006, 2011), however in the latter species it is reported that it may only have the humeral head (YOULATUS, 2000). In gibbons, this muscle is represented by four bellies that originate independently in the humerus, radius and ulna, and each one is directed towards a digit (MICHILSENS et al., 2009), although the radial belly can also be directed towards the digits II and III (HEPBURN, 1892) or a head for the digits IV and V (MICHILSENS et al., 2009).
Although in non-hominoid primates, where the Saguinus genus is located, the flexor digitorum superficialis muscle originates exclusively from the arm; and in hominoids also originates in the radius and ulna (DIOGO et al., 2012). However, this is not true in all cases, since in S. leucopus it is also originated in the tendon of the flexor digitorum profundus at the level of forearm, or in the case of the Sapajus genus, the radial origin can be found (AVERSI-F et al., 2006, 2011).
The innervations of this muscle by the median nerve as in S. leucopus also occurs in S. libidinosus (AVERSI-F et al., 2011) and H. sapiens sapiens (TESTUT & LATARJET, 1984; STANDRING, 2016), however in S. leucopus the branch of the median nerve to this muscle can penetrate it and communicate with the ulnar nerve (VÉLEZ-G et al., 2018). This communication can occur in H. sapiens sapiens in the flexor digitorum profundus muscle (CAETANO et al., 2016; NAKASHIMA, 1993), which allows us to suggest that the development of the lateral belly of the flexor digitorum superficialis muscle in S. leucopus is from the deep humeral head of the flexor digitorum profundus muscle. In S. leucopus this muscle was not supplied by the radial artery due to the lack of the radial head, which is in H. sapiens sapiens (TESTUT & LATARJET, 1984; STANDRING, 2016) and S. libidinosus (AVERSI-F et al., 2011), but in both species it is also supplied by the ulnar artery as in S. leucopus. The median artery also supplies this muscle in humans (TESTUT & LATARJET, 1984; STANDRING, 2016), but in S. leucopus the median artery was very well developed and provides a greater contribution than the ulnar artery.
Flexor digitorum profundus muscle (M. flexor digitorum profundus)
In C. goeldii and G. senegalensis five heads have been reported (OSMAN-H, 1959; STEVENS et al., 1977) lacking the cubital head of S. leucopus. Other primates differ from S. leucopus due to that they only have three heads: Humeral, radial and ulnar, as in C. albifrons (CRIBILLERO et al., 2009) and A. paniscus (YOULATUS, 2000). In others, there are only two heads: Radial and ulnar, as in C. olivaceus, A. seniculus (YOULATUS, 2000), M. mulatta, M. fascicularis, P. hamadryas, P. troglodytes, G. gorilla, P. pygmaeus (ACKERMANN, 2003; CHAMPNEYS, 1871; HEPBURN, 1892; KIMURA & TAKAI, 1970; PRIMROSE, 1900) and S. apella (YOULATUS, 2000), although in the latter (AVERSI-F et al., 2005), S. libidinosus (AVERSI-F et al., 2005, 2011), and P. troglodytes (CHAMPNEYS, 1871) the ulnar head has been reported as unique because the radial is considered as the flexor pollicis longus muscle, but this is also directed towards the digit II, thus it must be considered as a head of the flexor digitorum profundus muscle (DIOGO et al., 2012; VÉLEZ-G & CASTAÑEDA, 2016).
The fusion of the tendons proximal to the carpal canal in S. leucopus is also found in M. fascicularis (ACKERMANN, 2003; KIMURA & TAKAI, 1970) and G. senegalensis (STEVENS et al., 1977), while in other species this is described as a variable contribution by head for the digits, as in P. pygmaeus, P. troglodytes and G. gorilla, the ulnar head for the digits IV-V and the radial head for the digits I-III (ACKERMANN, 2003; HEPBURN, 1892). In great apes the tendon for the digit I may be very thin, vestigial or absent (PRIMROSE, 1900; DIOGO et al., 2012). The ulnar head sends tendons for the digits III, IV and V, and the radial (flexor pollicis longus muscle) for the digits I and III in S. apella (AVERSI-F et al., 2005), S. libidinosus (AVERSI-F et al., 2011) and P. troglodytes (CHAMPNEYS, 1871). In A. paniscus the radial head sends tendons for the digits II, III and IV, and the ulnar head for the digits IV and V (YOULATUS, 2000), which differ from the Ateles dissected by ACKERMANN (2003), who reports that the radial head sends tendon for digit II, and the ulnar sends for digits II-V. In S. syndactylus the muscular bellies and tendons can be found fused (MICHILSENS et al., 2009). In A. seniculus the radial head sends tendons for the digits I-IV, and the ulnar head for the digits III-V (YOULATUS, 2000), and in C. goeldii the humeral and radial heads send tendons for the digits I-III, and the ulnar heads for the digits III-V (OSMAN-H, 1959), being different to S. leucopus where the tendons of all the heads are fused and a separate contribution of each head was not observed.
The humeroradial complex (radial and humeral superficial heads) of S. leucopus is by homology the flexor pollicis longus muscle described in S. apella (AVERSI-F et al., 2005), S. libidinosus (AVERSI-F et al., 2011), and P. troglodytes (CHAMPNEYS, 1871), but the term flexor pollicis longus muscle is only applicable for H. sapiens sapiens, where it is only addressed for the digit I (STANDRING, 2016; FICAT, 1998; TESTUT & LATARJET, 1984) or in some occasions in gibbons (DIOGO et al., 2012; DIOGO & WOOD, 2012; HEPBURN, 1892).
In H. sapiens sapiens, the flexor digitorum profundus muscle is innerved by the ulnar nerve and anterior interosseous nerve (branch of the median nerve), and the flexor pollicis longus muscle only by the last nerve (TESTUT & LATARJET, 1984; STANDRING, 2016), which differs to S. leucopus, where an anterior interosseous nerve was not developed and instead we observed branches directly from the median nerve, being similar to that described by SWINDLER & WOOD (1973), who do not report the development of the anterior interosseous nerve in chimpanzees and bonobos, but differs to S. libidinosus where the latter nerve is reported (MARIN et al., 2009) and it is described that the innervation for this muscle in this species and S. apella is similar to that found in humans (AVERSI-F et al., 2005, 2011).
In S. libidinosus the arterial supply for the flexor digitorum profundus and flexor pollicis longus muscles is only reported by the ulnar artery (AVERSI-F et al., 2011), being different to S. leucopus where the arterial supply is greater through several arteries, which was similar to humans (TESTUT & LATARJET, 1984; STANDRING, 2016) if we take into account the two muscles and that the anterior interosseous artery is homologous to the caudal interosseous artery. However the contribution of the median artery in S. leucopus was important and always present for the humeroradial complex, different to the human, where only if this artery is well developed does it contribute to the flexor pollicis longus muscle (STANDRING, 2016). DUNLAP et al. (1985) report that the flexor digitorum superficialis and profundus muscles do not vary significantly in platyrrhines species, among them S. apella, C. goeldii and two of the Saguinus genus (S. geoffroyi and S. mystax), and ACKERMANN (2003) reports that the forearm muscles in Saguinus oedipus are the same as the human. However, our study demonstrates that the Saguinus genus may have high differences in S. leucopus, which authors such as AVERSI-F et al. (2005) and OSMAN-H (1959) had already demonstrated in S. apella and C. goeldii respectively. Therefore these differences are important to comparative anatomy and must be taken into account in clinical, surgical and orthopedic approaches in the caudomedial part of the forearm in primates (VÉLEZ-G & CASTAÑEDA, 2016).
CONCLUSION
The antebrachial digital flexor muscles in S. leucopus are highly developed, mainly the flexor digitorum profundus due to the presence of six heads distributed in the caudomedial part of the forearm, with broad origins from the medial epicondyle of the humerus, medial collateral ligament of the elbow, and the caudal and medial surfaces of the radius, ulna, and antebrachial interosseous membrane. In addition, it had developed a strong tendon product of the fusion of the all tendons of their heads, which together with the support of the flexor digitorum superficialis muscle for the digits II to V, and due to their adequate innervations and a high arterial supply, allow us to suggest that they have an anatomical adaptation that permits adequate strength to grab onto trunks and branches of the threes in vertical and horizontal quadrupedal positions, and also to tear the bark off the trees for feed off latex.














