INTRODUCTION
Tibial dyschondroplasia (TD) is a disorder of endochondral ossification in fast growing birds. This osteopathy affects the growth of the cartilage of the proximal tibiotarsus, the proximal metatarsal, and the femoral head affecting the longitudinal growth of the bones because of the interruption in the normal maturation of cartilage chondrocytes. It is produced by the inability of the pre hypertrophic chondrocytes to undergo terminal differentiation, forming an opaque plaque characterized by the presence of transitional cartilage, which is not reabsorbed and persists as a non-vascularized, demineralized soft tissue of irregular shape and size which extends from the growth plate to the metaphysis (Farquharson & Jefferies, 2000; Julian, 1998; Julian, 2005; Rath et al., 1998; Saif et al., 2008; Tselepis et al., 2000; Whitehead, 1995; Whitehead, 2009).
Various techniques are used to evaluate TD in broiler chickens including X-rays during ante-mortem examination, histological examination, immunohistochemical tests, microdensitometric analyzes, and macroscopic analysis of the lesion during the postmortem examination (Almeida Paz et al., 2005).
Diagnostic imaging of TD by means of a portable X-ray fluoroscope (lixiscope) is a non-invasive method performed on live animals resulting in an image of the bone in real time. The device uses low-intensity gamma rays and amplifies the image of the affected bone between 45,000 and 50,000 times. With this technique, used in field selection procedures, samples are scored differently and the disease is detected after 21 days of age. The Lixiscope often underestimates the severity of the lesion in cases of severe TD and does not detect small lesions of the disease (Almeida Paz et al., 2005; Takita et al., 1998; Thorp et al., 1993; Thorp et al., 1997).
The diagnosis of TD by histological examination is a reliable method to differentiate this disease from others such as rickets, hypocalcemia and other growth plate abnormalities (Thorp, 1994; Whitehead, 1995, 2009). This test evaluates the morphology and distribution of chondrocytes in the epiphyseal growth plate of the tibiotarsal joints, evidencing the degenerative alterations characteristic of tibial dyschondroplasia such as lack of calcification in chondrocytes, accumulation of avascular cartilage, absence of urate crystals and the presence of necrotic chondrocytes (Imik et al., 2012; Kapakin et al., 2019; Thorp et al., 1991). The measurement of proliferation or absence of cells is performed with the evaluation of the translucent area in which the rest, proliferative, hypertrophic or degenerative and calcification histological areas are, and which are essential to determine tibial dyschondroplasia in broiler chickens (Imik et al., 2012; Thorp, 1994). It is critical to consider that the diagnosis of TD through histological examination only, should be made by a specialist in histopathology after having processed the samples in a procedure that lasts approximately one week.
Immunohistochemical and microdensitometric analyses have also been applied in the TD study mainly to locate and quantify the expression of genes in birds with this disease. Through molecular tests, 1,630 Differentially Expressed Genes have been identified in animals with tibial dyschondroplasia (Tian et al., 2013; Zhang et al., 2019) and deficiency of the expression of the following factors, during this disorder, have been identified: transforming growth factor α and β (TGF-α, TGF-β); type X collagen; c-Myc protein, insulin-like growth factor-I (IGF-I); basic fibroblast growth factor; vascular endothelial growth factor (VEGF); proteoglycans and precursors of prostaglandins and glycosaminoglycans, as well as the decrease in the concentration of the core cartilage-specific proteoglycan or aggrecan (Farquharson et al., 1992; Mehmood et al., 2018; Saif et al., 2008; Thorp et al., 1993; Tselepis et al., 2000; Zhang et al., 2013; Zhang et al., 2018). Although the diagnosis with the use of molecular techniques for the identification and analysis of biological markers in pathological processes is efficient, when the classical methods are not effective, its use frequently raises the price of the diagnosis costs and specialized personnel is required for its execution and interpretation.
The macroscopic analysis is based on the observation and score of TD lesions present in the proximal tibial epiphysis where unilateral or bilateral thickening of the epiphyseal plate and the formation of an abnormal opaque mass of cartilage are observed (Almeida Paz et al., 2005; Imik et al., 2012; Kapakin et al., 2019; Thorp et al., 1991). Edwards & Veltmann (1983) on a scale, where the degree of TD is judged with a score between zero and three; zero means a normal cartilage and three means a large mass of hypertrophied cartilage, has been traditionally used. Based on the degree of thickening of the physis (Rath et al., 2004, 2007; Thorp et al., 1991, 1993, 1997) some modifications have been made to this scale used to calculate index and the incidence of TD. Despite its frequent use in the diagnosis of TD, macroscopic analysis is a highly subjective technique with variable results and low reliability because it is subject to the interpretation and expertise of the evaluator.
As objectivity is an essential element in diagnostic tests, even better if it is accompanied by low costs, quickness and easy execution, this research proposes a macroscopic analysis with a Quantitative Area Measurement Test including its respective scale for the diagnosis of TD in commercial lines of Gallus domesticus.
MATERIALS AND METHODS
All procedures performed in this experiment were endorsed by the Ethics Committee for Animal Experimentation at Universidad de Caldas, through Act No. 1 of 2014.
The animals were not subjected to processes that caused unnecessary pain or stress. The animals were immobilized considering the technical norms of handling and subjection of the animals, framed in Law 84 of October 27, 1989 (Colombian Statute of Protection of the Animals) (Mrad, 2006).
The experiment was carried out in the Laboratory of Nutrition and Poultry Health of the Faculty of Agricultural Sciences at Universidad de Caldas, Manizales, Colombia, with the endorsement of the University Ethics Committee for Experimentation with Animals to carry out all the procedures.
A total of 384 one-day-old Ross x Ross male broilers with an average initial weight of 43.9 ± 1.2 g were reared distributed in forty-eight 0.36 m2 vertical cages, equipped with a nipple type fountain and a trough. The animals received balanced feed of corn and soybean cake according to recommendations of NRC (1994) and Rostagno et al., (2011), plus a marginal dose of vitamin D (34.5 µg of 25-hydroxycholecalciferol/kg of food) to induce tibial dyschondroplasia. The lighting program with an intensity of 20 lumens/m2 was 24-hour light day, the internal humidity of the sheds remained at 57.5 ± 2.5%, and ventilation and temperature were regulated by an automatic control system according to the age of the birds.
Two random samples of 96 fowls each were taken (Figures 1, 2, 3, and 4). The first sample was taken at day 21 and the second at day 36 of chicken age for the clinical diagnosis of TD and to compare the two forms of measurement: Qualitative Score test (QST) and Quantitative Area Measurement (QAM) test. The birds were dazed by CO2 exposure and slaughtered. The right tibia was taken from each bird and a diagonal cut was made on the medial face of the head to expose the growth of the cartilage and to diagnose tibial dyschondroplasia by means of the TD qualitative scale with the participation of seven expert evaluators using the Edwards & Veltmann scale (1983). Then a photograph of the same samples was taken with a digital camera (Samsung DV150F, 16.2 MP and 5x optical zoom), mounted on a tripod at 20 cm. Subsequently, the areas were measured using the ArcGIS® program (version 9.3). The photograph of each sample was inserted in the ArcMap program, regardless of the measurement scale, and a total area between the epiphyseal articular cartilage area and the translucent area was delimited (Figures 1, 2, 3, and 4) corresponding to the 100% and, from this, the translucent area representing the tibial dyschondroplasia lesion was measured and its percentage score was given based on the tibial dyschondroplasia quantification scale, according to the QAM test (Table 1). Area measurement can also be done with software such as CorelDRAW, Publisher, AutoCAD and Solidworks.
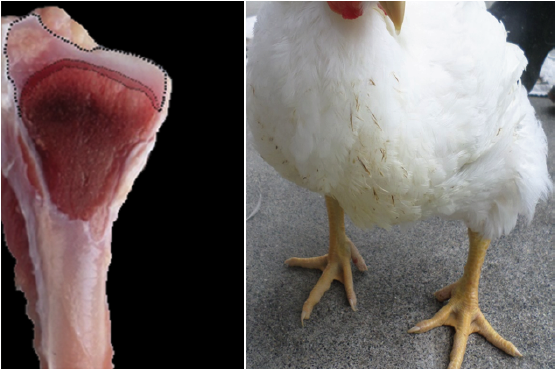
Figure 1 36 day old broilers and their diagonal cut on the medial face of the head of the right tibia. Normal degree: Inferior to 27% of translucent area space.
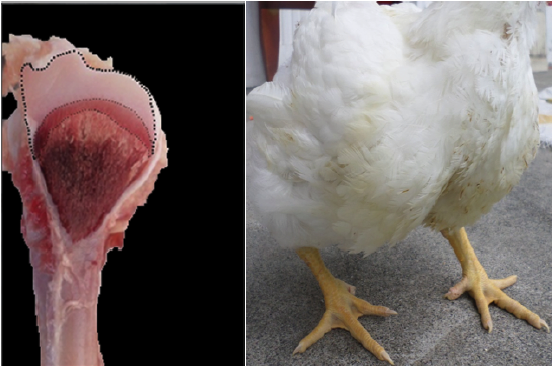
Figure 2 36 day old broilers and their diagonal cut on the medial face of the head of the right tibia. Low degree: Between 27 and 37% of translucent area space.
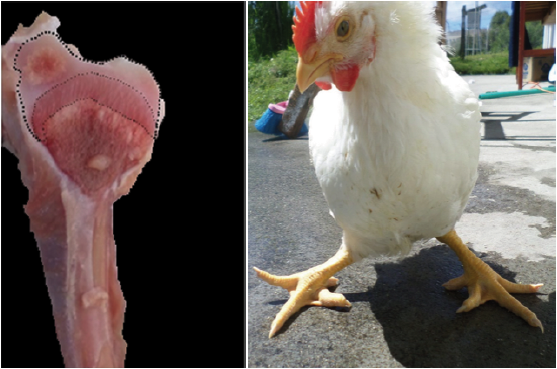
Figure 3 36 day old broilers and their diagonal cut on the medial face of the head of the right tibia. Medium degree: Between 38 and 47 % of translucent area space.
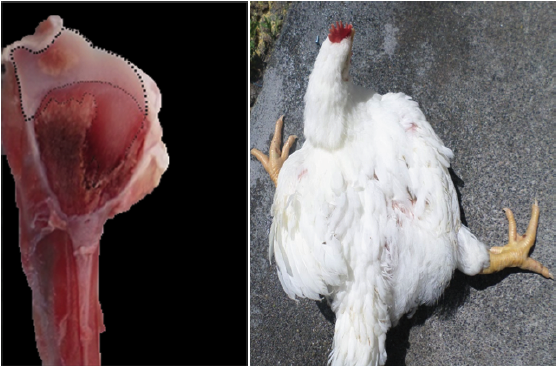
Figure 4 36 day old broilers and their diagonal cut on the medial face of the head of the right tibia. High degree: Superior to 47% of translucent area space.
Table 1 Tibial dyschondroplasia quantification Scale according to the Area Measurement System in broiler chicken.

Given that the test is purely qualitative, sensitivity, specificity, positive predictive value, and negative predictive value were calculated (Table 2) taking the QAM test as the reference test. In addition to that, the Spearman correlation test was applied to determine possible correlations between the two tests used in the experiment.
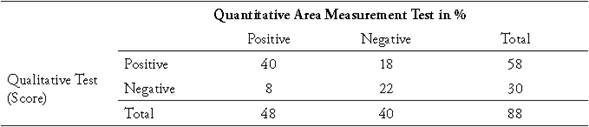
Table 2 Comparative table for diagnosis of tibial dyschondroplasia in broiler chickens at 21 days of age using Qualitative Score test and Quantitative Area Measurement test as a percentage.
To compare the results, only the diagnoses evaluated with score zero and one with the QST, which corresponded to those less than 27% or negative, and those between 27 and 37% considered as positive by the QAM test, were selected respectively. To reduce the error of subjectivity in the QST, and after the reading by seven experts of the same sample, the evaluation with the highest statistical mode was taken as reference.
RESULTS AND DISCUSSION
Tables 2 and 3 present the contingency tables of the diagnostic values of TD in broiler chickens of 21 and 36 days of age respectively, contrasting the QST versus the QAM test. In the 21-day-old chickens the QST had a sensitivity of 83%, while at 36 days of age the sensitivity was 100% meaning that, in older broilers, the macroscopic identification of TD becomes easier, possibly due to the increase in the size of the bone.
Table 3 Comparative table for diagnosis of tibial dyschondroplasia in broilers at 36 days of age using the Qualitative Score test and the Quantitative Area Measurement test in percentage.
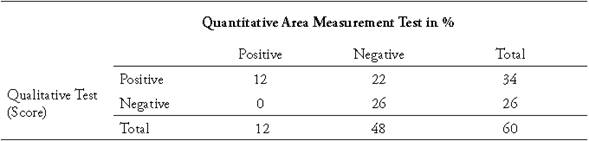
The QST had a specificity of 55% in chickens of 21 days of age, while at 36 days it was 54%. When determining the positive predictive value (PPV) of this test in 21-dayold chickens in the region studied, out of every 100 positive results, 60 chickens were positive and the remaining 40 were false positives. Meanwhile, when calculating the negative predictive value (NPV) with the QST, in 21-day-old chickens 80% of the negative cases did not actually have TD while the remaining 20% were false negatives. At day 36, 100% of the cases diagnosed as negative were indeed negative.
The results of the Spearman correlation test show that in 21-day-old chickens there is a significant positive correlation between the PCS and the MCA test with a rho of 0.40 (p <0.01) and in 36-day-old chickens with a correlation of 0.43 (p <0.01). This shows a low correlation for these techniques.
When comparing the two macroscopic diagnostic tests of TD in broiler chickens with or without the disease in degrees zero and one, the subjectivity of the QST test is already reflected. When it comes to classifying the evolution of the disease in degrees from zero to three, the discrepancy in the diagnostic results between the evaluators may be different since it depends only on the expert's judgment and criterion without a continuous quantitative reference of measure.
CONCLUSIONS
The Qualitative Score test for TD detection in 21-day old broiler chickens with diagnostic degrees between zero and one proved to be ineffective; there was a sensitivity of 83% and specificity of 55% when compared with the Area Quantitative Measurement test. Meanwhile, in 36-day-old broilers the same test showed a sensitivity of 100% and a specificity of 54%, verifying that the Quantitative Area Measurement test for tibial dyschondroplasia is an alternative quantification system for tibial dyschondroplasia in broiler chickens, which provides greater accuracy and objectivity against the conventional macroscopic diagnostic test. Similarly, the proposed method will allow research about new approaches of an etiology, pathogenesis and treatment of this disease.














