Introduction
The two-spotted spider mite Tetranychus urticae Koch is one of the most important pests in a wide range of outdoor and protected crops worldwide (Van Leeuwen et al., 2010). Strawberry plants in Brazil are frequently heavily infested and suffer severe damage from T. urticae. The mite mainly feeds on the underside of strawberry leaves, but it can also cause direct damage to fruit, flowers and growing tips (Oliveira et al., 2007).
Spider mites feed using a piercing-sucking process to remove cellular contents, which results in reductions of photosynthesis rates of strawberry plants (Kamelmanesh et al., 2010). Typical signs of spider mite feeding are death of plant cells; there is an initial yellow mottling as feeding continues, damaged areas coalesce, which causes scarring, bronzing, and drying of leaf tissue. This reduces the photosynthetic ability of the plant and, therefore, its vigor. If left uncontrolled, damage leads to stunted plant growth or eventual death.
Synthetic acaricides have been widely used to control T. urticae in different crops. Generally Broad-spectrum pesticides offer the promise of effective pest control although the risks associated with their incorrect use include residues in produce, adverse impacts on non-target beneficial organisms and the development of pest resistance to frequently used products (Mahmoud et al., 2002).
In Brazil, prophylactic applications of acaricides are widely used in commercial greenhouses to control spider mites on strawberry plants. Acaricides are usually applied at intervals of three or four days (Oliveira et al., 2007). However, increasing concerns on the impact of pesticides on the environment and human health have resulted in more restrictive governmental regulations regarding their use (Mostafalou and Abdollahi, 2012). Damage by spider mites has been reported even when acaricides were applied frequently. Consequently, alternative and sustainable methods are required for spider mite control in greenhouse strawberry production systems. Biological control with predatory mites is an effective alternative to chemical control of spider mites (McEwen and Haskell, 2013).
Two approaches have been developed for biological control of spider mites. First, the mass release of Phytoseiulus persimilis (Acari: Phytoseiidae) and other predatory mites (Simmonds, 1971; Kazak, 2008), and second, the conservation and increase of native populations of predators. The first option has been the most common strategy, but biocontrol of spider mites by native predators has also been attempted, mainly with other phytoseiid species (Waite, 1988; García-Marí and González-Zamora, 1999; Ferrero et al., 2011).
In Brazil, the native predaceous mite Phytoseiulus macropilis (Banks) was found in association with spider mites in strawberry crops in the state of Minas Gerais (Fadini et al., 2004; Oliveira et al., 2007). The presence of P. macropilis on strawberry plants likely originated from natural populations on native vegetation present near strawberry fields. Laboratory and greenhouse experiments showed that P. macropilis had a high rate of predation on T. urticae and could control spider mite populations in just a few days (Oliveira et al., 2007). Despite the presence of this predator in strawberry fields, crops are frequently treated with abamectin and dimethoate. This practice coincides with control measures used in other countries although high levels of resistance to both pyrethroids and avermectins have been reported in Europe (Rosario-Cruz et al., 2009), whereas the situation in Brazil has not been studied in detail to date.
Applications of acaricides in Brazil are timed to coincide with the appearance of spider mite populations after their initial detection by growers. At this time, P. macropilis populations are already present in strawberry fields so that predatory mites are exposed directly to spray droplets, acaricide residues on foliage and residues in and on their prey over the entire cropping cycle. However, sprayed plants continue to be damaged by spider mites and predatory mite populations are markedly reduced or absent in acaricide-treated crops (Fadini et al., 2004).
These observations suggest that the predator is more severely affected by acaricide treatments than by the pest. To examine this hypothesis, the mortality responses of both pest and predator were determined across a range of concentrations of abamectin and dimethoate. We also examined the influence of these compounds on the instantaneous rate of increase (ri) of predators and prey to determine likely population level effects of acaricide treatments. We use the results of these studies to suggest some alternative control strategies for spider mite populations in strawberry crops in Brazil.
Material and Methods
Adult P. macropilis and T. urticae were collected from strawberry fields in the southern state of Minas Gerais, Brazil and taken to the laboratory of Acarology at Federal University of Viçosa, Brazil. Spider mites were reared on arenas made of strawberry leaves that were placed on moist cotton pads on top of a damp sponge (3.0 cm thick) in a plastic Petri dish (15 cm x 25 cm x 5 cm). Predatory mites were also reared in these spider mites arenas using spider mites as prey. Arenas with predators and with phytophagous mites were kept in separate climatic chambers at 25 ± 1°C, 60 ± 5% RH and a 13 h: 11 h L: D photoperiod. Water was added when necessary to keep the cotton moist. Arenas were examined every day and when a high population of predators was observed, the predators were transferred to new arenas infested with the pest. Colonies of predatory and phytophagous mites were maintained for several months in the laboratory and were used in other studies.
Concentration-response bioassays. Concentration-response bioassays were carried out for both acaricides using adult female P. macropilis (4-5 days old), or T. urticae (14-15 days old) at the beginning of their reproductive period. Acaricides were sprayed on strawberry leaf disks (3.0 cm diameter) using a Potter tower. Spraying was carried out at 0.34 bar (= 3.44/104 kPa) with a 2.5 ml spray aliquot in line with the recommendations of the International Organization for Biological Control (Hassan et al., 1994). Technical grade acaricides (84% purity) were obtained from BASF and CHEMINOVA, both placed in Sao Paulo, Brazil. Acaricides were dissolved in water. Treatments were applied at a range of concentrations of 0.05 - 4.3 mg active ingredient (a.i.)/l. This range of concentrations was determined in preliminary studies to result in a range of mortalities between 3% and 70%. A control treatment involved leaf disks treated with water alone.The sprayed leaf disks and water-sprayed controls were air-dried for 1 h. Subsequently, 15 adult female predators or 15 adult female spider mites were placed on each disk. Ten replicates were prepared per acaricide concentration for each mite species. Treated leaf discs with mites were maintained at 25 ± 1°C, 60 ± 5% RH and a 13 h:11 h L:D photoperiod in a climatic chamber.
Additional strawberry leaf disks were sprayed with the same acaricide concentrations and groups of 200 spider mites were incubated on each disc. These mites were used as prey for P. macropilis during the experiment, to better reflect field conditions because in sprayed crops, predatory mite will only consume treated spider mites.
Mite mortality was assessed after 72 h exposure and mites were considered dead if they did not move after disturbing them with a fine brush. Concentration-mortality curves were estimated by Probit analysis (SAS Institute, 2004). For each acaricide, the index of differential selectivity was obtained by dividing the LC50 of spider mites by the LC50 of predatory mites, and 95% confidence limits were calculated.
Demographic bioassays. The instantaneous rate of increase (ri) was estimated using the equation ri =[ln(Nf/No )]/∆T, where Nf is the final number of living mites, No is the initial number of living mites and ∆T is the time interval (days) elapsed between the start and the end of the bioassay. The experimental procedures and acaricides were the same as those used for concentration-mortality bioassays with daily progeny assessments up to seven days following initial exposure. Either 15 adult female predators or 15 adult female spider mites were used per replicate. Here, 10 replicates in the experiment were also used.
The mites were kept in rearing chambers under the same conditions as the concentration-response bioassays. Groups of ∼30 spider mites were provided daily as food for the predatory mites. These spider mites were obtained from strawberry leaf disks sprayed with the same concentration as that of the leaf disks with predatory mites. The acaricide concentrations for the demographic assays were based on the previously obtained concentration-mortality curves. Controls were sprayed with water only. Regression analyses were performed to assess the effect of acaricide concentrations on the instantaneous rate of increase of each mite species.
Results and Discussion
Concentration-mortality studies involving acaricide residues on strawberry leaf disks revealed that abamectin was approximately 10-fold more toxic to pest and predatory mites than dimethoate (Table 1). Tetranychus urticae was less susceptible to both acaricides than P. macropilis (Table 1). Specifically, T. urticae was 2.6-fold and 4.2- fold less susceptible than P. macropilis to abamectin and dimethoate, respectively.
Table 1 Toxicity of abamectin and dimethoate to the predatory mite Phytoseiulus macropilis and its prey Tetranychus urticae.
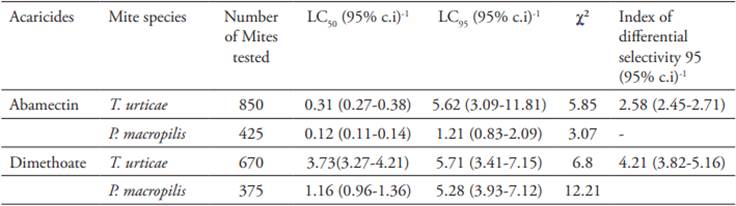
The instantaneous population growth rate of T. urticae and P. macropilis decreased linearly with increasing concentrations of both acaricides (Figures 1 and 2). Spider mite populations that were exposed to concentrations of abamectin around the LC50 (i.e., 0.31 mg a.i./l) showed positive values of ri (0.10 ± 0.08 day-1), indicating population growth after seven days of exposure to this acaricide (Figure 1). Extinction of spider mite populations occurred only at concentrations ≥ 0.42 mg a.i./l. Abamectin showed a more severe effect on the population growth of the predator, which declined at concentrations as low as 0.27 mg a.i./l (ri = -0.10 ± 0.04 day -1), a concentration at which spider mites experience positive population growth (ri = 0.12 ± 0.09 day-1) after seven days of exposure (Figure 1).
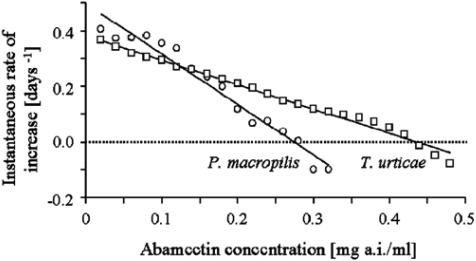
Figure 1 Instantaneous rate of increase (ri, day-1) of Phytoseiulus macropilis (open dots) (y = -1.8187x + 0.4414, r2 = 0.93) and Tetranychus urticae (open squares) (y = -0.8597x + 0.3543, r2 = 0.96) exposed to various concentrations of abamectin.
Results for dimethoate also revealed greater adverse effects for predatory mites than for spider mites. Predator extinction occurred at concentrations at or above 4.25 mg a.i./l (ri = -0.013 ± 0.001 day-1) whereas for spider mites, extinction occurred at 6.0 mg a.i./l (ri = -0.024 ± 0.001 day-1) after seven days of exposure (Figure 2).
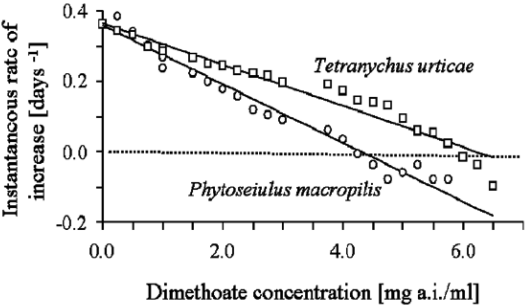
Figure 2 Instantaneous rate of increase (ri, day-1) of Phytoseiulus macropilis (open dots) (y = -0.0835x + 0.3587, r2 = 0.89) and Tetranychus urticae (open squares) (y = - 0.0586x + 0.3643, r2 = 0.86) exposed to various concentrations of dimethoate.
The spider mite T. urticae was more tolerant to the acaricides abamectin and dimethoate than its natural enemy, the predatory mite P. macropilis. These findings contrast with those of Kim and Yoo (2002), who reported that the acaricides bifenazate, acequinocyl, chlorfenapyr and fenbutatin oxide were much less toxic to Phytoseiulus persimilis Athias-Henriot adult females than to T. urticae adult females. Dekeyser et al (1996), also reported that bifenazate was highly toxic to phytophagous mites, such as Tetranychus, Eutetranychus, Oligonychus and Panonychus species, whereas it is harmless (<30% mortality) to predacious mites, such as the phytoseiids Amblyseius fallacis (Garman) and Galendromus occidentalis (Nesbitt).
However, studies in which predatory mites were more tolerant to acaricides than phytophagous mites do not correspond to the clear majority across most regions of the world.
Among the individuals who showed resistance to pesticides, natural enemies account for 2.2% of all registered cases worldwide (Aprd, 2016). One hypothesis is that natural enemies are more seriously affected than pest species because they may have less efficient detoxification mechanisms (Casida and Durkin, 2013). In addition, predators have greater exposure to pesticide residues than prey due to direct exposure to residues on the crop plant combined with exposure as they actively search for prey and the presence of residues in they prey they consume.Spider mites are well recognized for their tendency to develop resistance to commonly used acaricides. This is due to their short life cycle, frequent inbreeding and high fecundity. As repeated acaricide applications are required to control these pests, mites are continuously exposed to residues leading to the rapid development of resistance following one to four years of use of a given compound (Van Leeuwen et al., 2010).
The mites used in this study were collected from strawberry fields to which growers apply acaricides twice per week. Abamectin and dimethoate have been used here for at the past 10 years (H. Oliveira, personal observation), and strawberries are cultivated for eight months per year. Under this scenario acaricide resistance is likely to occur, as observed elsewhere. Indeed, resistance to dimethoate and abamectin has been reported in various T. urticae populations in Brazil (Sato et al., 2009).
Failures in chemical control of spider mites caused by resistance have been reported for various compounds, such as organophosphates, dicofol, organotins, hexythiazox, clofentezine and abamectin, only a few years after the introduction of such products (Adesanya et al., 2017). Furthermore, the frequent application of pesticides is likely to increase the concentration of residues in foods with obvious negative consequences for human safety and commercialization of fresh foods such as strawberries (Chowdhurya et al., 2013).
Concerns on the impact of pesticides on the environment and human health have stimulated research in alternative control measures. Considerable research efforts have been devoted to finding alternative strategies for suppression of T. urticae populations (Krishna and Bhaskar, 2016). To minimize the effect of broad-spectrum compounds on biological control agents and provide an ecological balance between pests and their natural enemies, these control strategies can be integrated to provide a biorational form of pest control. The use of selective pesticides that are compatible with natural enemies can be pivotal to the success of integrated pest management (IPM) programs (Abraham et al., 2013). Biological control of spider mites with predatory mite species such as Phytoseiulus spp. is now well established in some greenhouse crops (Gerson and Weintraub, 2012). Until recently, P. macropilis was not recognized in Brazil as a potentially effective biocontrol agent of T. urticae, and it was, therefore, not considered in decisions on acaricide applications. Indeed, acaricide applications are probably the main cause of predator absence on sprayed plants (Fadini et al., 2004; Oliveira et al., 2008), as confirmed in the present study.
Conclusions
The concentration-mortality and population-growth assessments presented here indicate that the use of abamectin and dimethoate at recommended concentrations cannot control spider mites, probably because of high levels of resistance to these compounds, they are harmful to predator mite populations through both direct mortality and negative population growth. As P. macropilis has a high potential to control spider mites, we believe that the control of spider mite populations may be improved by abandoning acaricide applications in strawberry fields in Brazil. They may also be further improved by grower education programs on techniques for the conservation of P. macropilis such as investing more in cultural control techniques, for example, keeping part of the old crop in the field and, thus, keeping colonies of predatory mites. Subsequently, these predators could be distributed on the new crops and, thus, initiate the preventive biological control and avoid the increase of future phytophagous mite populations.














