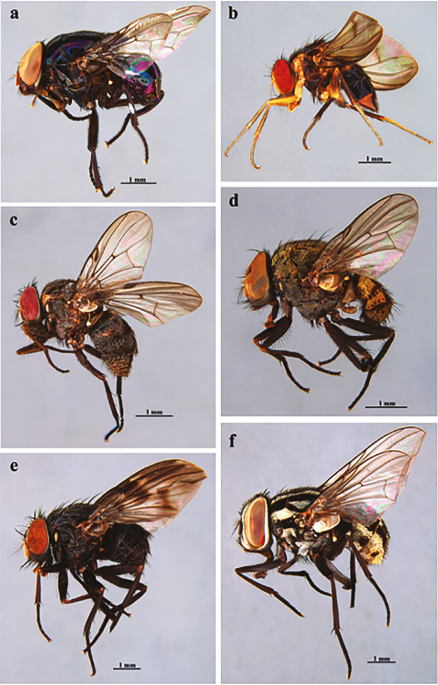
Introduction
Muscidae is one of the most diverse families of Diptera and exhibits a cosmopolitan distribution. Currently, there are about 5,200 described species distributed in 200 genera (Pape and Thompson 2013; Courney et al., 2017). The Neotropical region is home to approximately 850 species (Carvalho et al., 2005), of which 110 in 43 genera are found in Colombia (Pérez and Carvalho, 2016; Fogaça et al., 2019; Pérez et al., 2020).
Muscidae exhibit varied ecologies. Adults can be pollinators, saprophagous, coprophages, bloodsuckers or predators of other insects, they are present in most terrestrial habitats and a few in freshwater (Carvalho et al., 2005). Their larvae are mainly scavengers, which makes them relevant for ecosystems, since they contribute to the decomposition of organic matter and breed on decomposing animals, plant material, manure, and fungi (Skydmore, 1985; Couri et al., 2007). Muscidae include species with medical and veterinary significance because they act as mechanical vectors of pathogens that can affect both humans and animals and can cause myiasis while other species have forensic importance (Couri et al., 2007).
Some species show an important association with humans, a behavior known as anthropobiocenosis (Carvalho et al., 2005). The main cause of this behavior is the availability of resources that exist in these human-dominated environments (Carvalho and Couri, 2002). According to Nuorteva (1963), synanthropy is known as the association existing between certain animal species and humans, the nature of this relationship varies depending on factors such as species, geographic and climatic conditions of each region, as well as societal customs, which modify their environment according to their way of life. Gregor and Povolny (1958) divided the synanthropic behavior into three categories, based on the abundance of each species in a given ecological area: a) Eusynanthropy for species that are closely related to urban environments; b) Hemisynanthropy, for species that mainly inhabit semi-rural areas; and c) Asynanthropy for species that prefer forests in a good state of conservation, moving away from urbanized environments.
The Colombian Amazon has approximately 477,375 km2 that cover almost half of the continental shelf of the country with the largest area of forest in the country. However, these ecosystems remain poorly studied (Armenteras et al., 2006). According to IDEAM, (2020), during the year 2019, approximately 40.75% of deforestation in Colombia occurred in the Amazon; caused by the impact of oil exploitation, livestock, and agricultural expansion, including illicit crops. For the Neotropical region, studies have been carried out mainly in Brazil (Carvalho et al. 2005; Nihei and Carvalho, 2011) and Nicaragua (Nihei and Carvalho, 2009). In Colombia, Muscidae has been reported primarily in studies of forensic interest (Grisales et al., 2010) as well as in records and descriptions of the species of the family to catalogue the biodiversity of the country (Pérez and Carvalho, 2016). Although synanthropy has rarely been studied, Uribe et al. (2010) analyzed the degree of synanthropy of the Muscidae family in the municipality of La Pintada, Antioquia. For the Colombian Andean-Amazon region, studies on Muscidae have focused on forensic studies (Ramos-Pastrana et al., 2014; Ramos-Pastrana et al., 2018). The objective of this research was to analyze the synanthropic and ecological aspects of the Muscidae species in three habitat types with varying degrees of human intervention in the municipality of Florencia, Caquetá, Colombia in the Andean Amazon.
Materials and methods
The study was carried out in the municipality of Florencia, in the Department of Caquetá. This region has an average annual precipitation of 3,793 mm, the average temperature is 25 °C, and the mean annual relative humidity is 92% (Instituto Geográfico Agustín Codazzi [IGAC], 2010). The urban area took place in the municipality of Florence (01°36’19’’N, 75°36’28’’W) at 264; it has potable water, sewerage, and periodic garbage collection. The rural area, at 13 km (straight line) (01°29’55’’N, 75°39’25’’W) from the urban zone at 249 masl, corresponds to areas with pastures, crops, old stubble, relics of intervened forests, and low presence of settlers. The forest, at 14 km (straight line) (01°44’47’’N, 75°37’40’’W) from the urban area, at 716 masl, corresponds to a primary forest without the presence of houses.
The specimens were collected using Van Someren-Rydon traps, placed 1.5 m above ground and 50 m between them, baited with four attractants: human excrement, chicken viscera, fish, and decomposing onion. In each locality, four traps were installed, one per bait with approximately 150 grams of attractant. The study was carried out from March to August, sampling each month for periods of 48 continuous hours and removing the insects from the traps at 06:00 hrs. and 18:00 hrs., for a total of 288 hours of sampling per location.
The temperature, relative humidity, and precipitation data were provided by the meteorological stations of the Instituto de Hidrología, Meteorología y Estudios Ambientales (IDEAM) located near the sampling areas.
The collected material was taken to the Laboratorio de Entomología de la Universidad de la Amazonia. Only individuals belonging to the Muscidae family were selected. Subsequently, they were separated, sexed, quantified, and identified using the keys proposed by Carvalho and Couri (2002); Couri and Carvalho (2002); Marques and Couri (2007); Pamplona et al. (2016); Carvalho and Haseyama (2018). All individuals were deposited in the Colección del Laboratorio de Entomología de la Universidad de la Amazonia -LEUA-.
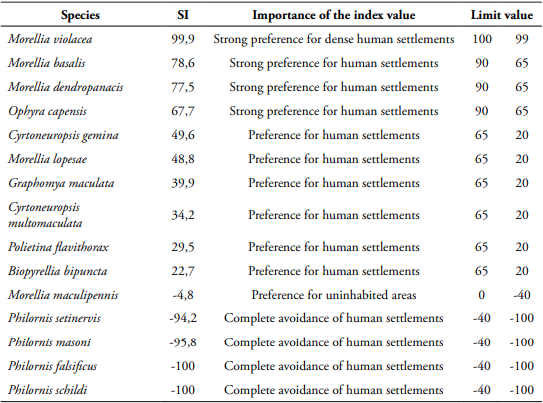
The synanthropic index (SI) was calculated using the methodology proposed by Nuorteva (1963), using the equation: SI=(2A+B-2C)/2; where A is the percentage of individuals of each species collected in the urban area; B the percentage of individuals of each species collected in the rural area; C the percentage of individuals of each species collected in the forest. This index varies between +100 and -100, where positive values indicate preference of flies for urban areas and negative values a preference for undisturbed areas. The intermediate values indicate different degrees of synanthropy. For the analysis, only species with 10 or more individuals were considered.
To evaluate the sampling effort, a species accumulation curve based on abundance was performed using the Chao1, Chao2, Jack1, and Jack2 estimators, using Estimates v.8 program for Windows (Colwell, 2006). To quantify the species richness based on the number of individuals, a rarefaction curve was constructed using the Biodiversity Pro v.2 program. Additionally, the Canonical Discriminant Analysis (CDA) was used to evaluate the relationship between the Muscidae species and the study area, bait, and time of collection. Using the statistical program RStudio v.3.6.2 (2016) to evaluate if there were significant differences in species richness and abundances between localities, sampling times, month, and type of attractant, the Kruskal-Wallis test (P=0.05) was performed using the statistical program InfoStat v.2018 and graphically represented using Box-plot boxes.
Results
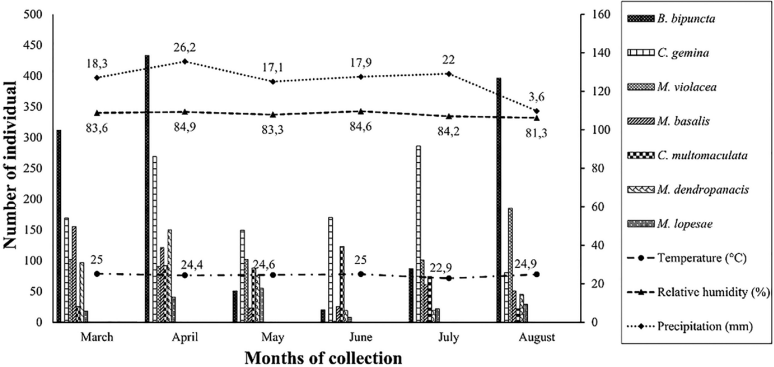
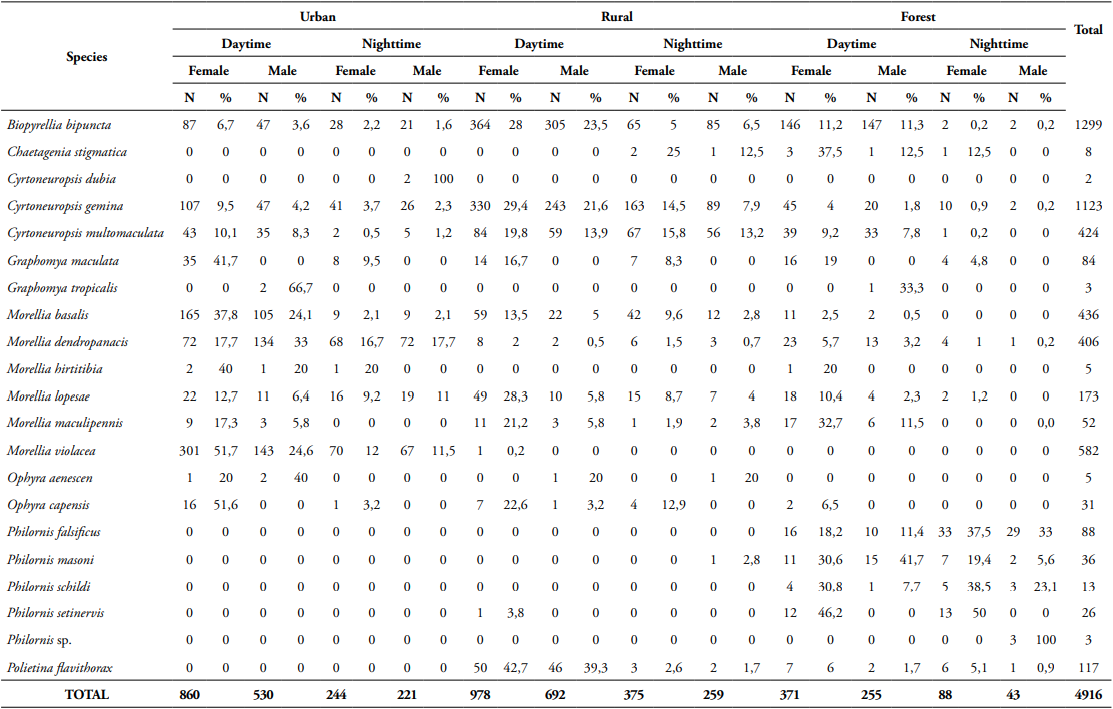
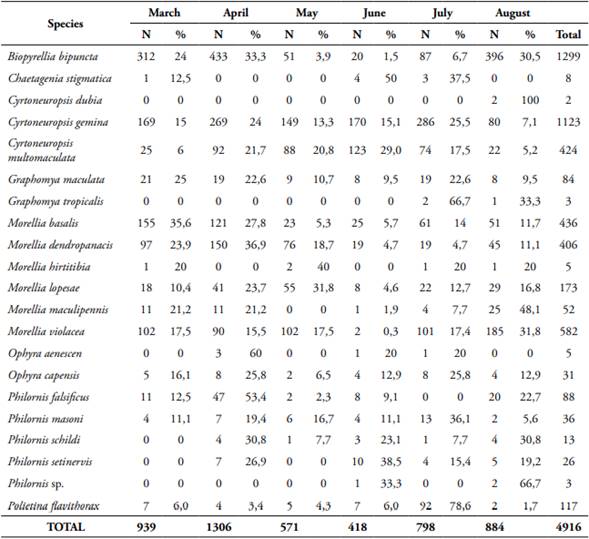
In total, 4,916 individuals of Muscidae that belong to eight genera and 21 species were collected, one genera and 11 species are new records for the country. Biopyrellia bipuncta (Wiedemann, 1830) was the most abundant, followed by Cyrtoneuropsis gemina (Wiedemann, 1830) and Morellia violacea Robineau-Desvoidy, 1830 (Fig. 1). Most species displayed diurnal activity, the females were more abundant (59.3%), the rural area had the highest number of specimens (49.9%), and the forest area had the lowest number (15.4%) (Table 1). Regarding attractant, fish was the most effective (50.8%), followed by human excrement (35.6%), chicken viscera (9.4%), and onion (4.2%) (Table 2), and the months of greatest activity were April (26.6%), March (19.1%) and August (18%) (Table 3).
Synanthropic index - Of the 21 species found, 15 had more than ten individuals (Table 4).
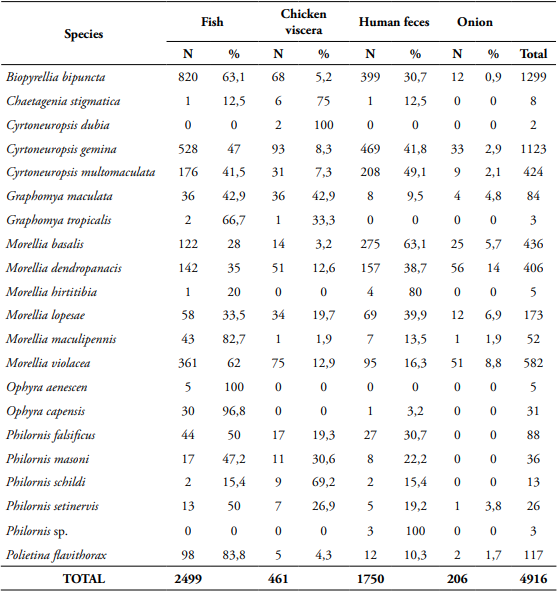
Morellia violacea: Neotropical distribution (Pérez and Carvalho, 2016). Exclusive to the urban area (99.8%), active mainly during daytime (76.5%) (Table 1, Figs 2, 3), shows a preference for fish (62%) (Table 2, Fig. 4). August was the month with the highest abundance (Table 3, Fig. 1) and the highest Synanthropic index (SI) 99.9; this species showed a strong preference for dense human settlements (Table 4).
Morellia basalis (Walker, 1853): Neotropical and Neartic distribution (Pérez and Carvalho, 2016). It was mainly abundant in the urban area (66.1%), and during daytime hours (83.5%) (Table 1, Figs 2, 3), showing a preference for human excrement (63.1%) (Table 2, Fig. 4). March was the month with the highest abundance (Table 3, Fig. 1). Its SI was 78.9, this species showed a strong preference for dense human settlements (Table 4).
Morellia dendropanacis (Pamplona and Couri, 1995): Distributed in Brazil, Panama, and Paraguay (Löwenberg and Carvalho, 2013). First record to Colombia. It was mainly found in the urban area (85.2%), and during daytime hours (62.1%) (Table 1, Figs 2, 3), shows a preference for human excrement (38.7%) and decomposed fish (35%) (Table 2, Fig. 4). They were most abundant in March and April (23.9%, 36.9%) (Table 3, Fig. 1). Its SI was 77.7; this species showed a strong preference for dense human settlements (Table 4).
Ophyra capensis (Wiedemann, 1818): Neotropical distribution (Carvalho and Couri, 2002). First record to Colombia. Its greatest abundance was in urban and rural areas (54.8%, 38.7%) during daytime hours (83.9%) (Table 1, Figs 2, 3), it was mainly attracted to decomposed fish (96.8%) (Table 2, Fig. 4). April and July were the months with the highest abundance (25.8% for each month) (Table 3). Its SI of 67.7 evidences a strong preference for dense human settlements (Table 4).
Cyrtoneuropsis gemina (Wiedemann, 1830): Widely distributed (Löwenberg and Carvalho, 2013). It was mainly found in the rural area (73.52%) during daytime hours (70.5%) (Table 1, Figs 2, 3), shows a preference for decomposed fish (47%) and human feces (41.8%) (Table 2, Fig. 4). July and April were the months with the highest abundance (25.5% and 24%) (Table 3, Fig. 1). Its SI of 49.6 demonstrates a preference for human settlements (Table 4).
Morellia lopesae (Pamplona, 1986): Distributed in Brazil (Löwenberg and Carvalho, 2013). First record for Colombia. It was collected mostly in rural and urban areas (46.8%, 39.3%), and during daytime hours (65.8%) (Table 1, Figs 2, 3), it was primarily attracted to human excrement and decomposed fish (39.9%, 33.5%) (Table 2, Fig. 4). May was the month with the highest abundance (31.8%) (Table 3, Fig. 1). Its SI was 48.8, demonstrating a preference for human settlements (Table 4).
Graphomya maculata (Scopoli, 1763): Cosmopolitan distribution (Couri and Carvalho, 2002). First record to Colombia. It was collected mostly in the urban areas (51.2%) during daytime hours (77.4%) (Table 1, Figs 2, 3), it was predominantly attracted to fish and chicken viscera (42.9%) (Table 2, Fig. 4). March was the month with the highest abundance (25%) (Table 3). Its SI was 39.9, which shows a preference for human settlements (Table 4).
Cyrtoneuropsis multomaculata (Stein, 1904): Neotropical distribution (Pérez and Carvalho, 2016). It was collected mainly in the rural areas (62.7%), and during daytime hours (69.1%) (Table 1, Figs 2, 3), it was primarily attracted to human feces and decomposed fish (49.1%, 41.5%) (Table 2, Fig. 4). June was the month with the highest abundance (29%) (Table 3, Fig. 1). Its SI was 34.2, evidencing a preference for human settlements (Table 4).
Polietina flavithorax (Stein, 1904): Neotropical distribution (Löwenberg and Carvalho, 2013). First record for Colombia. It was collected mostly in rural areas (86.3%), and during daytime hours (89.7%) (Table 1, Figs 2, 3), it was mainly attracted to decomposed fish (83.8%) (Table 2, Fig. 4). July was the month with the highest abundance (78.6%) (Table 3). Its SI was 29.5, demonstrating a preference for human settlements (Table 4).
Biopyrellia bipuncta (Wiedemann, 1830): Widely distributed in Central and South America (Pérez and Carvalho, 2016). It was collected mainly in rural areas (63%) during daytime (84.4%) (Table 1, Figs 2, 3), and it was attracted to decomposed fish (63.1%) (Table 2, Fig. 4), April and August were the months with the highest abundance (33.3%, 30.5%) (Table 3, Fig. 1). Its SI was 22.7, evidencing a preference for human settlements (Table 4).
Source: Own elaboration.
Figure 1 Monthly frequency of de seven most abundant species of Muscidae (represent 90.4%) collected in urban, rural, and forest areas, and behavior of environmental factors from March to August.
Morellia maculipennis (Macquart, 1846): Widely distributed in Central and South America (Pérez and Carvalho, 2016). It was collected primarily in forest areas (44.2%), during daytime hours (94.2%) (Table 1, Figs 2, 3), and it was attracted to decomposed fish (82.7%) (Table 2, Fig. 4). August was the month of greatest abundance (48.1%) (Table 3). Its SI was -4.8, which evidences a preference for inhabited areas (Table 4).
Philornis setinervis (Dodge, 1963): Distributed in Brazil (Löwerberg and Carvalho, 2013). First record to Colombia. It was collected mainly in the forest area (96.2%), with diurnal and nocturnal activity (50% both) (Table 1, Figs 2, 3), and it was attracted mostly to decomposed fish (50%) (Table 2, Fig. 4). June was the month with the highest abundance (38.5%) (Table 3). Its SI was -94.2, it showed complete avoidance of human settlements (Table 4).
Philornis masoni (Couri, 1986): Distributed in Brazil and Uruguay (Löwerberg and Carvalho, 2013). First record to Colombia. It was collected mostly in the forest areas (97.2%), during daytime (72.2%) (Table 1, Figs 2, 3), and it was mainly attracted to decomposed fish (47.2%) (Table 2, Fig. 4). July was the month with the highest abundance (36.1%) (Table 3). Its SI was -95.8, evidencing complete avoidance of human settlements (Table 4).
Philornis schildi (Dodge, 1963): Distributed in Costa Rica (Löwerberg and Carvalho, 2013). First record to Colombia. It was collected exclusively in the forest areas, during the day and night hours (Table 1, Figs 2, 3), it was primarily attracted to chicken viscera (69.2%) (Table 2, Fig. 4). April and August were the months with the highest abundance (30.8%) (Table 3). Its SI was -100, showing complete avoidance of human settlements (Table 4).
Philornis falsificus (Dodge and Aitken, 1968): It is widely distributed in Central and South America (Löwenberg and Carvalho, 2013). First record to Colombia. It was only recorded in the forest areas, the highest peak of activity was at night (70.5%) (Table 1, Figs. 2, 3), and it was primarily attracted to decomposed fish (50%) (Table 2, Fig. 4). April was the month with the highest abundance (53.4%) (Table 3). Its SI was -100, showing complete avoidance of human settlements (Table 4).
Species with less than 10 individuals
Chaetagenia stigmatica (Malloch, 1928): Distributed in Brazil (Löwenberger and Carvalho, 2013). First record to Colombia. It was collected in both forest and rural areas (62.5%, 37.5%), with diurnal and nocturnal activity (50% both) (Table 1, Figs 2, 3), it was mainly attracted to chicken viscera (75%) (Table 2, Fig. 4). June was the month with the highest abundance (50%) (Table 3).
Ophyra aenescens (Wiedemann, 1830): It is widely distributed (Pérez and Carvalho, 2016). It was collected in urban and rural areas (60%, 40%), and during daytime hours (80%) (Table 1, Figs 2, 3), it was exclusively attracted to decomposed fish (Table 2, Fig. 4). April was the month with the highest abundance (60%) (Table 3).
Morellia hirtitibia (Pamplona, 1986): Distributed in Brazil (Löwenberger and Carvalho, 2013). First record to Colombia. It was registered mainly in urban areas (80%), its highest peak of activity was during daytime (80%) (Table 1, Figs 2, 3), and it was primarily attracted to human excrement (80%) (Table 2, Fig. 4).
Graphomya tropicalis (Malloch, 1934): Neotropical distribution (Pérez and Carvalho, 2016). Recorded mostly in urban areas (66.7%), exclusively during daytime (Table 1, Figs 2, 3), and it was attracted mainly to decomposed fish (66.7%) (Table 2, Fig. 4).
Philornis sp. was collected exclusively in the forest areas, only showed activity at night (Table 1, Figs 2, 3), and it was exclusively attracted to human excrement (Table 2, Fig. 4).
Cyrtoneuropsis dubia (Snyder, 1954): Neotropical distribution (Pérez and Carvalho, 2016). Two male individuals were collected, exclusively in the urban area, and at night (Table 1, Figs 2, 3), it was baited with chicken viscera (Table 2, Fig. 4).
Table 1 Absolute and relative frequency, collection time, and sex of the specimens of Muscidae collected in the three sampling areas in the municipality of Florencia, Caquetá.
Source: Own elaboration.
Table 2 Absolute and relative frequency of the species of Muscidae captured with each of the attractants in the three sampling areas of the municipality of Florencia, Caquetá.
Source: Own elaboration.
Table 3 Absolute and relative frequency of the specimens of Muscidae collected in the months of March/ August in the municipality of Florencia, Caquetá.
Source: Own elaboration.
Table 4 Synanthropic Index (SI) for Muscidae species in the municipality of Florencia, Caquetá.
Source: Own elaboration.
Species accumulation curve - Although the curve does not reach the asymptote, the addition of species during the final surveys was low, only until sampling 18 it was possible to collect one more species. Thus, the species found in the three localities corresponded to 88.8% of the expected species with the Jack and Chao estimators (Fig. 5A).
Rarefaction curve - The forest area showed the highest species richness (n=18), and the lowest abundance, while the rural area had fewer species and greater abundance, the urban area was intermediate in both aspects (Fig. 5B).
Kruskal-Wallis test - Concerning the abundance according to the collection sampling times, significant differences were found (P=0.002) (Fig. 6A).
In terms of abundance per attractant, there were no significant differences between decomposing onions and chicken viscera (P=0.9798), and between human excrement and decomposing fish (P=0.4045). However, there were significant differences between decomposing onion and human excrement (P=0.0277), and between human excrement and chicken viscera (P=0.0363). Decomposing fish showed significant differences concerning the attractant decomposing onions and chicken viscera (P=0.001) (Fig. 6B). No significant differences were found regarding the months of sampling in terms of abundance (P=0.85684) (Fig. 6C).
Regarding richness, chicken viscera and human excrement did not show relevant differences (P=0.83557). However, decomposing onion presented major differences regarding human excrement (P=0.001), decomposing fish (P=0.001), and chicken viscera (P=0.01177); as well as between decomposing fish, human excrement (P=0.001), and chicken viscera (P=0.001) (Fig. 6D). Finally, there was no significant difference in attractant between night and day (P=0.83249) (Fig. 6E).
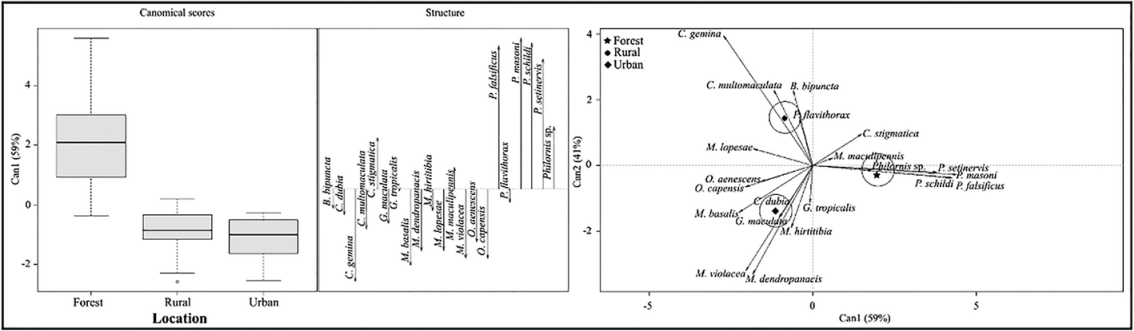
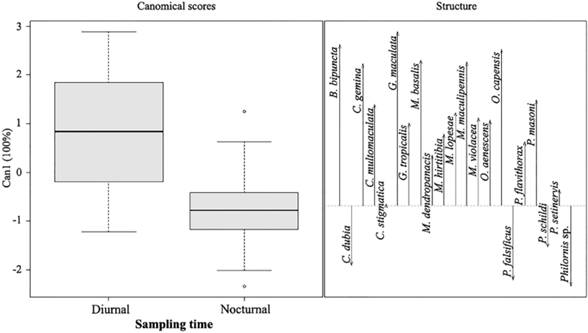
Canonical Discriminant Analysis (CDA) - The rural and urban areas showed similarity in the abundance and richness for the species C. gemina, M. basalis, O. capensis, M. violacea, M. lopesae, M. dendropanasis, O. aenescens, C. multomaculata, G. maculata, C. dubia, B. bipuncta, M. hirtitibia, P. flavithorax and G. tropicalis (Fig. 2). The forest presented significant differences with the rural and urban areas, mainly in the abundances of C. stigmatica, M. maculipennis, P. falsificus, P. masoni, P. schildi, P. setinervis, and Philornis sp. (Fig. 2). The time did not show similarity in the abundance and richness of the species, only Philornis sp., P. falsificus, C. dubia, and P. schildi were collected mainly during nocturnal hours; the other species were collected mostly during diurnal hours (Fig. 3).
Source: Own elaboration.
Figure 2 Canonical correlations analysis of Muscidae species in urban, rural, and forest areas.
Source: Own elaboration.
Figure 3 Canonical discriminations analysis of Muscidae species for each day/night sampling hours.
The decomposed fish and human excrement attractants showed similarity in the abundance and richness of the species O. capensis, C. gemina, B. bipuncta, O. aenescens, M. maculipennis, C. multomaculata, P. falsificus, M. basalis, M. violacea, P. flavithorax, P. setinervis, P. masoni, M. lopesae, M. dendropanasis, M. hirtitibia, Philornis sp., G. tropicalis (Fig. 4). The chicken viscera and decomposing onion attractants showed similarity in the abundance and richness of the species C. stigmatica, P. schildi, C. dubia, and G. maculata (Fig. 4).
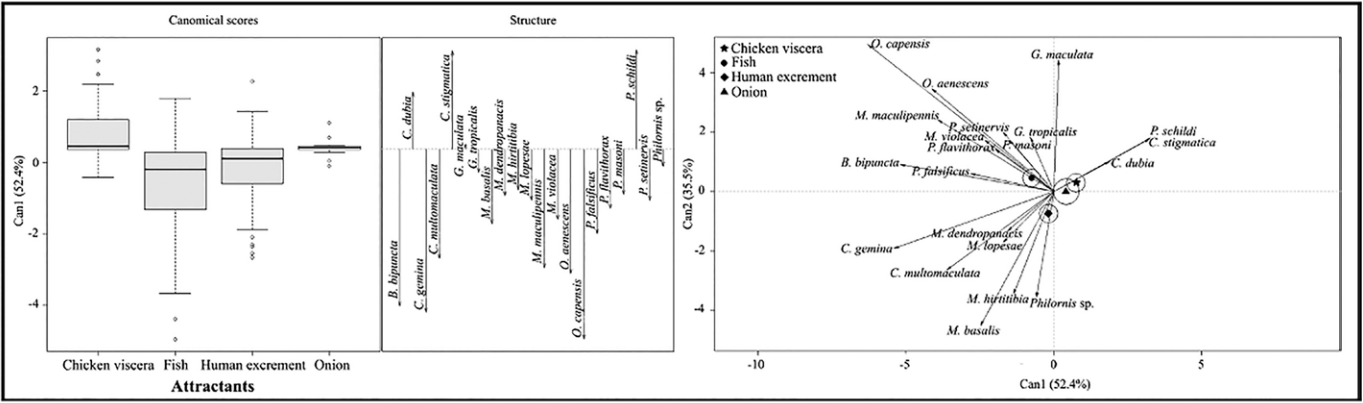
Source: Own elaboration.
Figure 4 Canonical discriminations analysis of Muscidae species for the four attractants used.
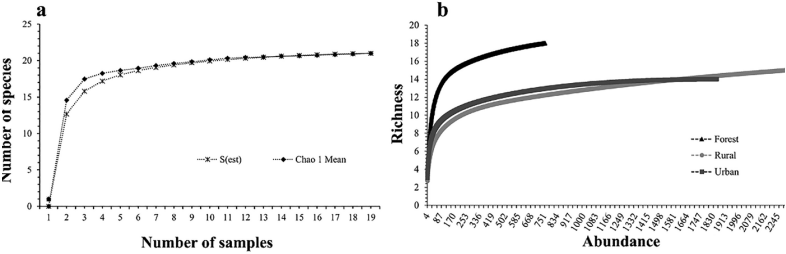
Source: Own elaboration.
Figure 5 A: Accumulation curve of Muscidae species collected in Florencia, Caquetá, Colombia. B: Rarefaction curve of Muscidae species collected in Florencia, Caquetá, Colombia.
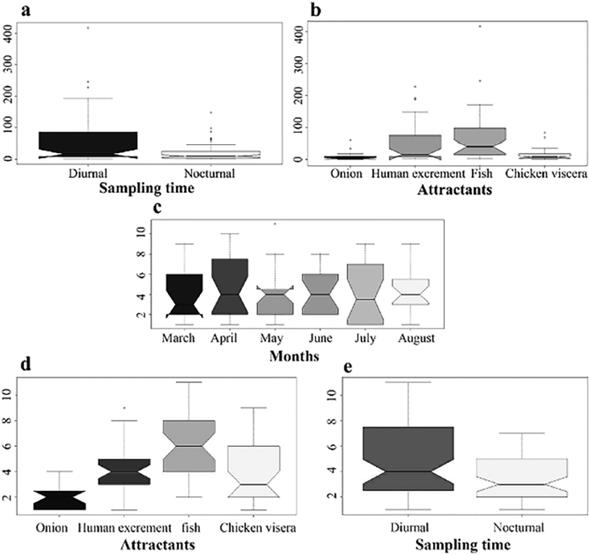
Source: Own elaboration.
Figure 6 A: Abundance of Muscidae species for each day/night sampling period. B: Abundance of Muscidae species for each attractant used. C: Abundance of Muscidae species from March to August. D: Muscidae species richness for each attractant used. E: Muscidae species richness for each day/night sampling period.
Discussion
In this research, 4,916 individuals from eight genera and 21 species were collected. These results are similar to those obtained by Uribe et al. (2010) who captured 5,726 individuals from 13 genera and 19 species, but significantly different from those obtained in Chile by Figueroa-Roa and Linhares (2004) with 2448 individuals, from six genera and six species.
Biopyrellia bipuncta was the most abundant species, with greater abundance in the months with higher rainfall and humidity (Appendix 1, Fig. 7a). This species showed a strong preference for decomposing fish and human excrement, confirming what was reported by Carriço et al. (2015), who mentioned its association with decomposing animal tissue. B. bipuncta was collected more frequently in rural areas, reaching a low synanthropic index, and showing a preference for human settlements. These conditions could indicate this species could be considered as an important vector of diseases in human settlements in rural areas of the municipality of Florencia. These results differ from that reported by Uribe et al. (2010) who described for the municipality of La Pintada this species obtains a high synanthropic index, showing a preference for uninhabited areas, but it agrees with the reports by Koller et al. (2004) and Luiz et al. (2012) who indicated this species is associated with areas that have low human intervention.
Cyrtoneuropsis gemina was the second most abundant species, it showed an increased abundance during the months with higher precipitation (April and July). This species was predominantly collected in rural areas and evidenced a preference for human settlements and fish, reaching an intermediate synanthropic index. These results differ from that reported by Uribe et al. (2010) who described their greatest abundance in urban areas, showing a strong preference for human settlements, and it was collected mainly in human feces. For this study, this implies a potential use as an indicator species in forensic entomology in the rural areas of the municipality of Florencia.
Morellia violacea was the third most abundant species and had an increased abundance in the months with the lowest precipitation (August) (Appendix 1, Fig. 9). It was collected mainly in the urban areas and had the highest synanthropic index. It was mainly attracted to fish and human feces, supporting previous findings by Skidmore (1985), who also reported the genus associated with these two substrates. Based on our findings we suggest this species could be used as an indicator of forensic entomology in the urban areas of the municipality of Florencia.
The next most abundant species were M. basalis, M. dendropanacis and C. multomaculata, the first two showed a preference for urban areas and were mainly attracted by excrement and fish, this agrees with the findings by Uribe et al, (2010) but it is in contrast with Costacurta et al. (2003) who reported that M. dendropanacis was collected in forest areas. Rodríguez-Fernández et al. (2006) reported that M. basalis and M. dendropanacis were collected with fish. C. multomaculata showed a preference for rural areas and was mainly attracted to excrement and fish, the resource preference found in this study could suggest the potential for C. multomaculata to act as a vector of pathogens in rural areas in the municipality of Florencia.
Morellia lopesae and P. flavithorax both showed a preference for rural habitats. They were primarily attracted to decomposing fish and human excrement, which is in line with the reports by Luiz et al. (2012), who described that P. flavithorax was collected in fish in a synanthropy study in Brazil.
Graphomya maculata and O. capensis displayed a preference for urban environments and were mainly attracted to decomposing fish and chicken viscera, agreeing with Carvalho and Pont (1998) who reported they are related to urban areas and attracted to human feces, which is in contrast with the results of this study. Bourel et al. (2004) reported that O. Capensis was collected in association with decomposing bodies in confined situations in northern France. Additionally, Cavallari et al. (2015) reported the species in decomposing human bodies in urban areas of São Paulo, confirming its preference for decaying animal tissue.
Philornis falsificus, P. masoni, P. schildi, P. setinervis, and Philornis sp. evidenced a preference for forest areas and had a necrophagous behavior. These results agree with Carvalho et al. (2005) and Couri et al. (2007) who reported that P. masoni, P. setinervis, and P. falsificus in their larval stage act as a parasite for birds, which continues to feed on the host after death.
Morellia maculipennis showed a preference for forest areas and were mainly attracted to fish, the resource preference found in this study could suggest the potential for M. maculipennis to act as a vector of pathogens in rural areas in the municipality of Florencia.
Ophyra aenescens, M. hirtitibia, G. tropicalis, C. dubia, C. stigmatica were the least abundant species, but no less important for this study. O. aenescens was collected exclusively in fish and mainly in urban areas, according to what was reported by Couri et al. (2009), who associated these species with decomposing bodies. However, it contrasts Patitucci et al. (2013) who collected the largest number of individuals in rural areas.
Morellia hirtitibia is associated with urban areas and it is attracted to human excrement. This habitat and resource preference contrasts what the reports by Luiz et al. (2012) who recorded the genera mainly in forest areas. However, the resource preference found in this study could suggest the potential of M. hirtitibia to act as a vector of pathogens in urban areas in the municipality of Florencia.
Cyrtoneurosis dubia was collected exclusively from an urban area and collected only with chicken viscera, according to the reports by Nihei and Tolezano (2015) who described that C. dubia is associated with decomposing animal tissue in urban areas of Brazil.
Graphomya tropicalis was captured in the urban and forest areas, mainly attracted to fish (Appendix 1, Fig. 8). Chaetagenia stigmatica showed a preference for forest areas and it was mainly attracted to chicken viscera, this suggests this species might be used as a forensic indicator in the forests of the municipality of Florencia.
Conclusions
Muscidae is an important Diptera family found in Colombia and the Amazon region, of which little ecological and taxonomic information is known, despite knowing species of this family with ecological, medical, and forensic importance. This study provides new relevant information on the synanthropic index, behavioral ecology, habitat, and resource preferences, as well as nictemeral activity for eight genera and 21 species of Muscidae that occur in urban, rural, and forest areas in the municipality of Florencia, Caquetá, Colombia.
Although previous studies focused on Diptera including species of Muscidae have been carried out in Colombia and the Andean-Amazon region, knowledge of biology, behavior, food, and habitat preferences are still insufficient. Therefore, it is important to carry out more research to fill the knowledge gaps. Moreover, given that the changes associated with anthropogenic activity, including climate change, might affect species differently depending on their vulnerability and shape, the fauna present in certain habitats, synanthropic species may be particularly vulnerable to change.