Introduction
Cognition (from the Latin cognoscere = knowledge) is defined as the ability to perceive, react, process and understand, store and retrieve information, make decisions and produce appropriate responses (Cauchoix et al., 2018), which, in the field of human psychology, is associated with intelligence (Ardila, 2011). When it comes to animal behavior, we talk about cognitive performance because tests are performed in different conditions to the ones used in humans (oral or written language) (Schönpflug, 2001). In vertebrates, cognitive assessments usually consist of tests that relate directly to their learning capacity; for example, the assignment of new tasks is almost always related to the solving of food access problems (Schubiger et al., 2020) . However, test performance is affected not only by brain and processing capacity but by body condition, age, and personality, amongst other so-called confounding variables (Kamil, 1987).
Van Horik (2017) found that birds with good body condition were more motivated to participate in food reward trials (Van Horik et al., 2017). Regarding personality, proactive individuals tend to learn associations faster than less curious ones, as in the case of male Poecilia reticulata (Poeciliidae) whose most audacious individuals learned to associate an orange or green ring with their food (Dugatkin and Alfieri, 2003). Shyness and neophobia are personality traits that can generate bias when performing a test, more cautious birds take fewer risks and will therefore take longer to solve the test (Shaw, 2017). However, this does not necessarily mean that birds will demonstrate poor cognitive performance, they just need more time to analyze the environment and make decisions (Madden et al., 2018). This fear is a factor that can be reduced by allowing for habituation to either the researcher or the test apparatus and is called “latency time”, which is the time it takes for the individual to approach a new device or person in the feeding area (Greenberg, 1990).
Given traditional beliefs, the demonstration of cognitive abilities of birds has generated strong discussion. For many years, birds were believed to lack “intelligence” (Oswald, 1964) due to its reduced brain size (Thorpe, 1956). Recently, however, numerous studies have evaluated cognitive abilities and found that certain groups, especially parrots and crows, can attain the cognitive level of primates when using tools (Hackett, 2020). For instance, they can solve problems related to finding difficult to access food, remembering the location of food (even after a few months), and being aware of the expiration date of such foods, among other numerous tasks (Dally et al., 2006).
Natural environments highly disturbed by human activities result in urbanization. This leads to the growth of cities and, as a result, an increase in the number of civil constructions in previously was a rural environment (Uttara et al., 2012). These land use changes generate new selective pressures, which influence behavioural responses and population densities of the organisms living there. For some bird species, environmental changes represent both threats [e.g., possible window collision, pollution, habitat deterioration (Patten and Kelly, 2010)] as well as advantages [e.g., provision of abundant food resources throughout the year, new nesting sites, more stable environmental conditions and less exposure to wild predators (Shochat et al., 2010)]. Under these conditions, the population density of some birds has been shown to be 30% higher in urban than in rural areas (Møller et al., 2012) suggesting behavioural adaptations.
Avian cognition is considered to play an important role in the adaptation that must occur in highly disturbed environments. Accordingly, studies that seek to understand and evaluate how cognitive capacity favours behavioural plasticity in response to the demands of an anthropogenic environment become increasingly relevant (Griffin et al., 2017). In a literature review of Neotropical passerines, innovative behaviour was documented in more than 200 species, including Quiscalus lugubris and Loxigilla barbadensis that live in the city and take advantage of the resources urban life offers. Q. lugubris picks up concentrated dog food with its beak and soaks it in cups of water before eating it. Whereas, L. barbadensis steals and opens bags of sugar that people leave in cafeterias (Lefebvre et al., 2016).
The degree of adaptation of a species to a certain extent reflects and depends upon their habitat. For example, if populations living in habitats with different degrees of urbanization are evaluated, it is possible to predict that they will differ in behavioural aspects such as shyness, learning speed and reward association (Valcarcel and Fernández-Juricic, 2009). In a comparative study between urban and rural populations of L. barbadensis, it was found that the urban population fed faster after human disturbance and performed better on the problem-solving test than their rural counterparts (Lefebvre et al., 2016). Similarly, when comparing problem solving in Parus major in urban vs. forest habitats, urban birds solved the test faster than those located in the forest (Preiszner et al., 2017). In contrast, in female Acridotheres tristis, urban individuals were slower than rural individuals when processing a visual discrimination test (Federspiel et al., 2017).
One of the most common and abundant species in urban and suburban environments in Valle del Cauca is the thraupid Sicalis flaveola flaveola, commonly known as Safron Finch. It is a granivorous bird that inhabits savannahs with bushes, scattered trees, meadows and gardens (Remsen et al., 2021). In recent decades the distribution of S. flaveola in Colombia has changed: it was initially reported in the Caribbean area and eastern plains but, in a relatively short time, has managed to occupy the middle and lowlands of the Andean region (Hilty et al., 2001). On the Atlantic Coast S. flaveola individuals were kept as caged birds and it has been speculated that some may have escaped or been released; then, they successfully colonized areas such as the geographic valley of the Cauca River (Hilty et al., 2001). Another possible scenario is that deterioration of the pristine habitat of the Darién area allowed the gradual passage of individuals to new areas (Álvarez-López, personal communication, 2015). S. flaveola is one of the most successful species in the city of Cali, Valle del Cauca, but so far, aspects related to its adaptive capacity in terms of its behaviour are unknown. For this reason, the present study aims to generate a baseline on the cognitive performance of S. flaveola to examine whether differences in cognitive performance (problem solving and discrimination) are related to behavioural characteristics (shyness, neophobia), body characteristics (body condition and sex) and the habitat that individuals occupy (degree of urbanization). The degree of adaptation of a species to a certain extent reflects and depends upon their habitat. For example, if populations living in habitats with different degrees of urbanization are evaluated, it is possible to predict that they will differ in behavioral aspects such as shyness, learning speed and reward association (Valcarcel and Fernández-Juricic, 2009). We predict that individuals who are faster at solving problems and learning to discriminate will be less fearful of participating in cognitive tests, that is, less shy, neophobic and will have better body condition, than those who are slower.
Methods
Study area
The study was carried out in areas of the cities of Santiago de Cali (3°27′00″N, 76°32′00″W; 995m) and Jamundí (3°15′39″N, 76°32′22″W; 975 m), located in the Cauca River Valley, department of Valle del Cauca, Colombia. Average annual temperature and rainfall in Cali and Jamundí are 25°C and 1,471 mm (Alcaldía Santiago de Cali, 2018) and 25°C and 1,706 mm, respectively (IDEAM, 2015). The climatic conditions of both cities are classified as tropical dry forest according to the Holdridge life zones (Espinal, 1967) (Alvarado-Solano and Ospina, 2015) and present different degrees of urbanization. Cali has a total population of 2,445,281 inhabitants and a population density of 4,382.05 inhabitants/km² (DANE, 2020) while Jamundí has 124,586 inhabitants and a population density is 168.1 inhabitants/km² (Alcaldía de Jamundi, 2021).
Field work and cognition
Between July 2019-February 2020 and 2022, systematic tours were carried out in the two cities to find the birds’ communal roosts; 10 mist nets (12 m long, 5 m high, 30 mm mesh eye) were installed between 17:00 and 19:00. Captured S. flaveola adults were kept in individual cages and were provided with horizontal perches, water and ad libitum food (yellow corn and commercial seeds) supplemented with vitamins and calcium. Individuals were visually but not acoustically isolated from their conspecifics. Morphometric measurements were recorded for all: beak length, wing chord, tarsus length and rectrices with a vernier caliper (± 0.01 mm) and body mass with a digital scale (± 0.01g). Molecular sex determination was carried out from a blood sample (10ul) taken from the brachial vein and the birds were ringed with coloured bands on the tarsus, for their individual recognition. All tests were conducted between 07:00 and 16:00. Prior to testing, birds were deprived of food for one hour, and when they had finished ad libitum food and water were provided until the next day. These activities were approved under the framework of the ethics committee of the S. flaveola macroproject.
Shyness and habituation. On the third day of captivity, a shyness test was performed to assess the degree of fear or rejection to the experimenter. A container with food was introduced into the cage and with a video recorder, we measured how long it took for the bird to access the food once the person moved out of sight. At the end of this period the bird remained alone for 20 min, and in order to examine habituation to human presence, the procedure was repeated five times.
Obstacle removal test. This test consisted of measuring the time and number of attempts required to move an obstacle that prevented access to food. A foamy lid (diameter 7.5 cm) was attached to the container with a single point of contact, so that it could rotate if pushed by the bird (Fig. 1B). The container had translucent walls, which allowed its content to be observed from the outside. The test had four levels of difficulty (Fig. 1B): level 1, the container was presented with the lid completely open. If the birds fed from the container within a period of 10 minutes, it was considered a success. In case of failure, the test was repeated a maximum of two times; level 2, the container was partially closed. If the bird failed (did not feed), it was returned to the previous level until it succeeded; level 3, the container was almost closed. In this case, the space did not allow feeding without carrying out the removal task, by rotating the lid of the container with the beak; level 4, the container was presented closed and when the individual managed to move the lid and eat, it was considered a success. This test was presented to individuals until they passed and was applied up to 129 times. The first exposure to the learning device was the test for neophobia. If the bird fed during two or more repetitions at each level, that is, several 10-minute intervals, it was ready to move to the next level.

Photos: Manuela Barreiro-Padilla.
Figure 1 A) Difficulty levels in obstacle removal test: 1. Open container, 2. Semi-open, 3. Slightly open, 3. Closed. B) Apparatus used in the colour discrimination test.
Colour discrimination test. The test consisted in identifying the location of the food by selecting the container with the corresponding colour. To start the discrimination test it was first necessary for birds to complete the obstacle removal test. During this test, birds were simultaneously presented with two containers, one green and one white, arranged on the same surface and 20 cm apart (Fig. 1C). During the first stage, both containers were presented with plenty of food and with the lids open for ten minutes. The colour of the container the birds first chose to feed on was recorded and we assumed this was the preferred colour.
Statistical analysis
Shyness, neophobia and habituation. After reviewing the videos, data were obtained for shyness and neophobia (dependent variables) using as independent variables: a) body condition, defined as the residuals of the simple linear regression model between the commissure and their body mass; b) sex and c) degree of urbanization, the origin of the population, in this case Cali or Jamundí. The assumptions of normality and homoscedasticity in the residuals of the linear regression models were verified using the Shapiro Wilk (Shapiro, 1990) and Barlett (Salkind, 2012) tests, respectively, a Multiple Linear Regression Model (MLR) was used in the analysis to find if neophobia or shyness were affected by body condition, sex and degree of urbanization. To measure whether there was habituation, Spearman’s correlation test was performed between the latency time and the number of test repetitions. In addition, whether the latency time, that is, the time used by the bird to feed, was correlated with factors such as body condition, sex, or degree of urbanization (Weaver et al., 2017).
Removal. To evaluate the cognitive performance in the obstacle removal test, a Linear Mixed Model (LMM) was used, the dependent variable was the learning speed (VelAp), defined as the time in seconds birds needed to achieve success until the last level. The independent variables were shyness, neophobia, sex, body condition (condcorp), degree of urbanization (urban), level of the test (level), number of repetitions or attempts to achieve success (rep) and the random factor included was identity of the individual (id). The data of the dependent variable was transformed to base ten logarithms, to fulfill the assumption of residual normality. The generated models were chosen using the Akaike criteria (Akaike, 1974)) and Bayesian information-BIC (Schwarz, 1978).
Whether the total number of attempts required to learn to remove the obstacle (dependent variable) was affected by shyness, neophobia, sex, individual body condition, degree of urbanization and test level of difficulty (independent variables) was also evaluated by using a Generalized Linear Mixed Model (GLMM) with Poisson error distribution. Identity of the individuals was included as a random factor.
Discrimination. In order to assess whether the learning speed of colour discrimination (dependent variable = Y) varied according to individual traits (shyness, neophobia, sex, body condition, degree of urbanization, level-number of attempts in the test, removal learning speed; as independent variables), a Linear Model Mixed (LMM) was used with the identity of the individual as a random factor. The assumption of normality (of the residuals) was fulfilled transforming variable (Y) to the base ten logarithm.
Whether the number of repetitions required by the birds to learn to discriminate the location of food by colour and the number of failed approaches (AcerF) (dependent variables) were related to individual traits through two GLMMs with Poisson error distribution and individual identity as a random factor was also studied. As the variables, number of repetitions and failed approaches presented a high correlation (Spearman r = 0.939, p < 0.001), the two models carried out were compared with the purpose of choosing one that best explained the phenomenon by means of the Akaike- AIC and Bayesian information-BIC. In both models, the variable (Y) was transformed to a logarithm of base ten, which allowed the assumption of normality of the residuals to be fulfilled, and an analysis of variance (ANOVA) was carried out. For the statistical analyses, the lme4 (Bates et al., 2021), nlme (Pinheiro et al., 2021), nortest (Gross Juergen, 2015) packages were used, in the statistical environment R and Rstudio (Team Core R, 2021).
Results
A total of 28 adult individuals of S. flaveola were captured (16 from Cali and 12 from Jamundí).
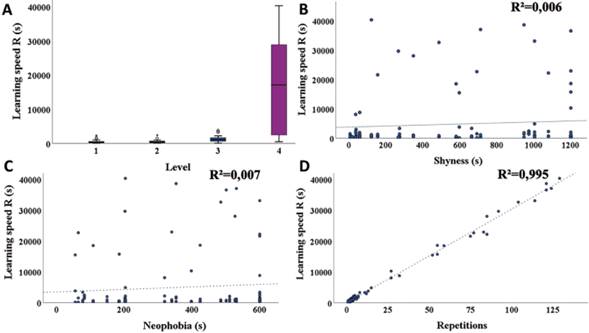
Shyness. The expression of shyness in S. flaveola in the presence of humans in their environment varied between 8 and 1080 s (328 s ± 203.94) and was not related with the co-variables body condition, sex, or degree of urbanization according to the MLR (Table 1).
Table 1 Multiple Linear Regression Model with interaction between factors for shyness, as a response variable, in Sicalis flaveola, Colombia; Post-model analysis of variance. Degrees of freedom = 11.
| Variable | DF | SS | MS | F | p |
|---|---|---|---|---|---|
| urban | 1 | 104.218 | 104.218 | 2.342 | 0.154 |
| condcorp | 1 | 2.665 | 2.665 | 0.059 | 0.811 |
| sex | 1 | 15.028 | 15.028 | 0.337 | 0.572 |
| urban:condcorp | 1 | 24.651 | 24.651 | 0.554 | 0.472 |
| urban:sex | 1 | 68.767 | 68.767 | 1.545 | 0.239 |
| condcorp:sex | 1 | 5.517 | 5.517 | 0.124 | 0.731 |
| urban:condcorp:sex | 1 | 10.825 | 10.825 | 0.243 | 0.631 |
| Residuals | 11 | 489.358 | 44.487 |
Source: compiled by authors.
Neophobia. Individual neophobias varied between 56 and 601s with a mean of 517s ± 431.18. The MLR showed that the degree of neophobia was not affected by the variables considered in the analysis (Table 2), meaning that the fear expressed by individuals of new objects in their environment is not directly related to body condition, sex, or degree of urbanization of the city.
Table 2 Multiple Linear Regression Model with interaction between factors for neophobia, as a response variable, in Sicalis flaveola, Colombia. Post-model analysis of variance, Degrees of freedom = 23.
| Variable | DF | SS | MS | F | p |
|---|---|---|---|---|---|
| Urban | 1 | 0.555 | 0.555 | 0.921 | 0.347 |
| Sex | 1 | 0.858 | 0.858 | 1.425 | 0.244 |
| condcorp | 1 | 0.250 | 0.250 | 0.415 | 0.525 |
| urban:sex | 1 | 0.070 | 0.070 | 0.116 | 0.735 |
| Residuals | 23 | 13.858 | 0.602 |
Source: compiled by authors.
Habituation. S. flaveola showed no evidence of habituation as interaction with humans increased, nor was there a correlation with neophobia, shyness, or degree of urbanization in the city of origin (Table 3).
Table 3 Spearman’s correlation for habituation to humans (latency time) with number of repetitions of the interaction test and other traits of Sicalis flaveola individuals (n = 28); in southwestern Colombia.
| Variable | Estimate (R) | p |
|---|---|---|
| Number of test trails | 0.055 | 0.521 |
| Body condition | 0.022 | 0.798 |
| Sex | 0.041 | 0.630 |
| Urban | -0.172 | 0.042 |
Source: compiled by authors.
Cognitive performance
A total of 258 cognitive performance tests, 129 obstacle removal tests and 129 discrimination tests were performed on 28 individuals.
Removal. 25 out of 28 (89.29%) individuals successfully completed the obstacle removal test (11 from Jamundí and 14 from Cali) and required an average of 4,039 ± 8,027 s to successfully complete the test.
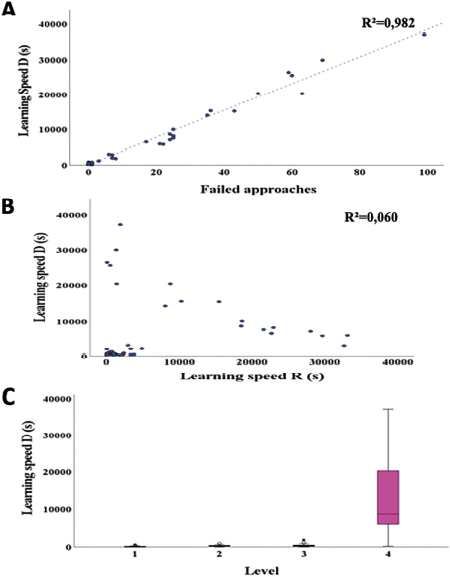
The MLR calculated for cognitive performance analysis related to the obstacle removal test, showed that neophobia (F0.05, 1, 21 = 13.767, p = 0.001), shyness (F0.05, 1, 21 = 5.823, p = 0.025), number of test repetitions (F0.05, 1, 71 = 393.519, p < 0.001), and test level (F0.05, 3, 71 = 22.815, p < 0.001) affected learning speed (Annex A, Table 4). This means the more fearful of humans and foreign objects an individual was, the longer it took for them to solve obstacle removal problems. Similarly, the greater the degree of difficulty in the cognitive test, the longer the individuals required to solve it. Finally, the individuals who took the longest to learn were those who had to practice the most (Fig. 2).
Source: compiled by authors.
Figure 2 Significant variables in the obstacle removal test for Sicalis flaveola with learning speed as a variable. A) Difficulty level, B) shyness, C) neophobia and D) number of repetitions.
The number of times individuals required to repeat the test to achieve success, according to the GLMM, was significantly affected by the number of failed approaches to the cognitive battery (F0,05,1 = 186.223, p < 2.2 x10-16), test level of difficulty (F0,05,3 = 203.394, p < 2.2 x10-16) and interaction between sex and level (F0,05,3 =14.339, p = 0.002478). In other words, the more mistakes they made, the greater the number of test repetitions that were required. It was also found that the highest number of repetitions was recorded more frequently in the fourth level, where females performed fewer repetitions than males (Fig. 3).
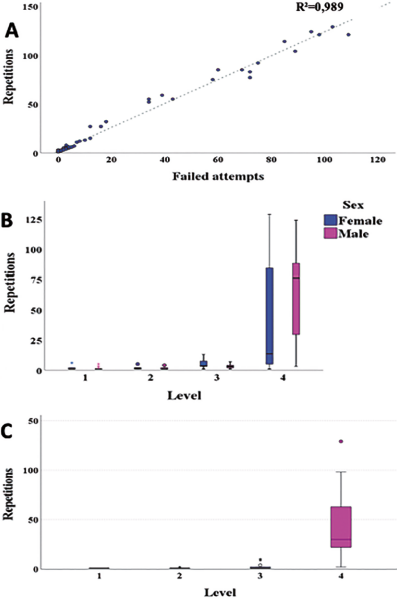
Source: compiled by authors.
Figure 3 Significant variables in the obstacle removal test for Sicalis flaveola with repetitions as a variable. A) unsuccessful approaches, B) level of difficulty according to sex and C) level of difficulty.
Discrimination. Out of 25 individuals who managed to pass the removal test and took the colour discrimination test, 11 (44%) associated the colour with the reward (4 from Jamundí and 7 from Cali) and required an average of 3,541 s ± 7.65 to complete it.
The fastest individual required 478 s while the last one to successfully complete the test took 38,116 s. We found that the number of failed approaches during the test (F0.05, 1.48 = 320.944, p < 0.001), obstacle removal speed (pre-test) (F0.05.1, 48 = 40.430, p < 0.001) and difficulty level (F0,05,3,48 = 11.477 p < 0.001) significantly influenced the colour discrimination learning speed. This means the more failed attempts required to solve the test, the longer it took to be successful (Fig. 4A). In relation to obstacle removal speed, two groups of individuals with different performances are observed: 1) those who learned the fastest to remove the obstacle, were also the fastest to learn to discriminate and 2) individuals who required more time to learn to remove the obstacle took less time to learn to discriminate; meaning there is an inverse relationship (Fig. 4B). Regarding the level of difficulty, the fourth level was the one that required more time investment by birds (Fig. 4C). The number of times that the individuals required to repeat the discrimination test to achieve success was not affected by any of the variables analyzed, according to the GLMM and the respective ANOVA (Annex B, Table 5).
Discussion
Our results reveal that with S. flaveola shyness and neophobia are not influenced by place of origin (degree of urbanization), body condition or sex and, although these characteristics affect learning speed to remove obstacles, this is not the case with discrimination. Our results were contrary to what has been reported in the literature, where the degree of urbanization is a factor that affects shyness and neophobia (Tryjanowski et al., 2016) (Sol et al., 2011; Lefebvre et al., 2016), in S. flaveola these behavioural traits did not present any association with the degree of urbanization, body condition or sex. For example, in Loxigilla barbadensis, neophobia, audacity and the ability to open a container were evaluated, individuals from urban areas were less shy and more neophobic than those from rural areas (Lefebvre et al., 2016). A similar trend was also observed in Milvago chimango, where urban individuals were less neophobic than rural ones and performed better (Biondi et al., 2021).
However, Sol et al. (2011) found that urban populations of Acridotheres tristis were less neophobic than suburban populations, probably because individuals from suburban populations are exposed to predation by raptors, and consequently, are more fearful than their conspecifics from urban raptor-free areas (Griffin and Boyce, 2009). Bokony et al. (2012) carried out personality tests with different populations of Passer domesticus along an urbanization gradient, they found that neophobia did not decrease in urban environments, something similar occurs with Phalcoboenus chimango where individuals from both populations did not present differences in neophobia (Solaro and Sarasola, 2019); probably because birds that occupy new spaces remain cautious when exploring them and, therefore, they are more likely to survive. What happens with S. flaveola is that even in urban environments, individuals show fear when around humans. Even though birds eat corn people place on the pavement, S. flaveola does not accept nearby passers-by and will fly away from the resource each time anyone comes close to that feeding area. This fear may be because birds evaluate the risks of predation when approaching a new environment (Sol et al., 2011) and in cities, cats, dogs, and vehicles constitute a permanent threat to fauna (Seymour et al., 2020) [Cruz-Bernate personal observation]. The S. flaveola strategy of staying alert can be considered adaptive as it allows the species to survive in this environment.
Body condition, an estimate of energy status, has been shown to be an important factor in motivation when interacting with new objects because it will determine the energy budget the individual can dedicate to solving challenges to obtain food (Seaman et al., 2008). Thus, the relationship is proportional between body condition and the probability of participating in a problem-solving test, as evidenced in Phasianus colchicus (Van Horik and Madden, 2016; Van Horik et al., 2017). The birds in this study had good body condition, since all the individuals presented an index close to the ideal balance, as in the experiment by Jakob et al. (1996); which most likely contributed to the absence of significant differences in participation.
Regarding the influence of sex, the evidence is diverse. In some cases where males tend to be more dominant than females, for example, in Zenaida aurita, it was shown that there is a differential participation between the sexes, with the males having priority access (Boogert et al., 2019). Something similar was also found with male Phasianus colchicus, who are larger and faster growing than females (van Horik et al., 2017). A rapid rate of body growth is known to be a good indicator of motivation when interacting with tests (Ruiz-Gomez et al., 2011). On the other hand, sexual dimorphism by body size of S. flaveola is subtle although there are significant differences between males and females in wing length, rectrices, primarysecondary distance and bill width (Espinosa et al., 2021) participation by sex may not be biased. In addition, the way in which this experiment was performed, with isolated individuals, rules out that the fear of participating in the tests was mediated by dynamics of social or sexual dominance. These results were similar to those in Taeniopygia guttata where no differences were found in the participation of cognitive tests (Lambert et al., 2021).
Cognitive performance
Sicalis flaveola was successful in the obstacle removal test and most of them solved the assigned task; indicating that the species can learn through training. Personality traits can mark differences in cognitive performance between individuals of the same species (Goumas et al., 2020). For instance, less neophobic juveniles of Melopsittacus undulatus can access food faster by removing an obstacle inside the seed container (Medina-García et al., 2017). Furthermore, exposure to anthropogenized environments affects one of the ways in which cognitive performance is manifested: behavioural flexibility when searching for food and shelter (Biondi et al., 2020); important to survival and adaptation to the urban habitat (Lee and Thornton, 2021). Finally, body condition is another factor associated with cognitive performance (van Horik et al., 2017). Shaw (2017) reported that Petroica longipes individuals with a higher body condition index required fewer attempts for a reverse learning task than their conspecifics, with a lower index.
Shyness significantly influenced the time required to remove the obstacle; the shyest individuals recorded a longer latency time. These results are similar to those found by (Ducatez et al., 2019) with Quiscalus lugubris, in a test similar to the present study, where shyer individuals needed more attempts in the obstacle removal test, consistent with the hypothesis of Sih and Giudice (2012), which posits that shy individuals adopt a slow, low exploratory behavioural strategy, where they accumulate fewer resources, but take fewer risks and gain in survival.
In the same model, we found that neophobia also affects learning speed in the removal test (Annex B); the least neophobic birds needed less time to successfully solve the task. This result is consistent with another study with Q. lugubris, where the challenge was to peck only in specific places that allowed it to run a hinge to obtain food; the least neophobic birds had the best performance and they were considered as the most innovative (Overington et al., 2011). Boogert et al. (2008) reported there is a negative correlation between innovation and neophobia since the process that an animal goes through when overcoming neophobia, approaching a new stimulus, and trying a new technique for feeding are similar (Webster and Lefebvre, 2001). It can therefore be stated that both species, S. flaveola and Q. lugubris, have more adventurous and daring individuals who solve challenges that would not be found in their natural environments. As Szabo, Damas-Moreira and Whiting (Szabo et al., 2020) found, the ability of a species to survive in new areas may be related to behavioural flexibility and brain size in birds. In New Zealand, for example, species with greater innovations and large brains are the most successful invaders, since they adopt new strategies of foraging and association with humans to obtain benefits (Sol et al., 2002).
Discrimination test results confirm that S. flaveola can distinguish by colour and show the influence of failed approaches and removal learning on discrimination learning speed. Here the results vary regarding the obstacle removal task, since this test requires other types of skills, not only motor but also association (Van Horik et al., 2019) and discrimination learning generally requires more time to complete and collect information (Sih and Giudice, 2012). Failed attempts play an important role in learning speed, because birds have to spend more time training and trying to find the solution to a given problem (Chittka et al., 2003) The relationship between learning speed in the removal and colour discrimination test was positive for a subgroup of individuals; but for the other group the relationship between both tests was inverse (Fig. 4B); birds of the first group showed similar behaviour in both tests, which according to Burkart, Schubiger and van Schaik, (2017) could indicate that performance was determined by overall cognitive ability and not by specific domains of information processing. The other group results could be explained by the speed-accuracy trade-off hypothesis, which occurs when some animals spend more time making more precise and safe decisions while others are faster and more imprecise (Guillette et al., 2011). When individuals face a discrimination task, there is an inverse relationship between test solving speed and their accuracy; thus, if more time is invested in solving the problem, greater precision is achieved, or the task is completed in less time, but at the cost of making more errors (Dyer and Chittka, 2004). These decisions occur in several ecological domains, for example, in the detection of prey by predators, the selection of flowers by pollinators, and in spatial exploration strategies where locating resources and nesting sites is vital for survival (Chittka et al., 2009).
There was no effect of shyness and neophobia in the discrimination test, suggesting that after undergoing the obstacle removal test, birds possibly habituated to both the experimenter and the test apparatus. These results are similar to those found by Ducatez et al. (2015) with Q. lugubris. Our findings provide guidelines to evaluate in the future if with S. flaveola, the ability to occupy new habitats is influenced by its cognitive performance, as it has been for other colonizing species (Sol et al., 2002), since behavioural flexibility when it comes to obtaining food, is an advantage when they need to exploit new resources and compete against other species (Magory Cohen et al., 2020). For example, in populations of Passer domesticus, individuals that originated from an actively invasive population approached and consumed new foods faster than birds from an established population (Liebl and Martin, 2014). However, it is not possible to affirm that the cognitive capacities of S. flaveola are a decisive factor for its survival and success in new habitats, it is necessary to carry out tests in wildlife that resemble challenges that are associated with its natural history (Pritchard et al., 2016).