Introduction
Thrips are pernicious pest species in numerous of crops worldwide (Lewis, 1997; Teulon et al., 1993). Although there are plenty of records of infestations and economic damage inflicted by trips in neotropical countries (Alves-Silva & Del-Claro, 2010), their management strategies have been comparatively more studied in temperate regions. In Colombia, the species Neohydatothrips signifer (Priesner, 1932) is considered as a key pest species of the yellow passion fruit Passiflora edulis (Sims, 1818) as it can inflict damage in up to 95% of the vegetative terminals and 75% of the flower buds (Salamanca Bastidas et al., 2010; Santos et al., 2012b). This species was originally described in Mexico (Priesner, 1932), but it has also been recently recorded in other South American areas, including Colombia (Lima and Mound, 2016; Santos et al., 2012b). Besides attacking yellow passion-fruit crops, it is also known to infest Brickellia argyrolepis B. L. Rob. (Asteraceae) (Lima and Mound, 2016).
Previous studies have proposed a monitoring protocol (Santos et al., 2012a) which provides an economic threshold for N. signifier in yellow passion crops of 6-10 thrips per terminal bud depending on the mean temperature (Santos et al., 2012b). However, no study to date has attempted to establish the effectivity of sticky traps equipped with semiochemicals to control this pest species. The necessity for using kairomone-baited sticky traps to collect thrips arises because of the thigmokinetic behavior (i.e., occupation of narrow spaces within plant organs) that these insects exhibit. This condition imposes a challenge for the visual recognition of these insects and their damage (Childers, 1997). Consequently, kairomone-baited sticky traps are suitable for early detection of thrips and can be even helpful for mass trapping thrips during an infestation depending on trap density, as shown by previous studies (Kawai, 1990; Lim et al., 2013; Lim and Mainali, 2009; Sampson and Kirk, 2013). In some crops with significant affectation by thrips (i.e., peanut, tobacco and tomato), the control strategy was based mainly on systemic and foliar insecticides (Mouden et al., 2017; Riley et al., 2018). However, the cryptic behavior of thrips hampers the action of insecticides (i.e., they are harder to reach) (Kirk et al., 2021). Furthermore, it is well known that misuse of synthetic pesticides in agricultural environments may disrupt needed ecological interactions (i.e., biological control by natural enemies) and induce insecticide-resistance evolution (Desneux et al., 2007; Gutiérrez, 2020; Mahmood et al., 2016; Mouden et al., 2017). Additionally, the utilization of broadspectrum insecticides is not advisable in yellow passion fruit crops as P. edulis is strictly self-incompatible and relies heavily on bee pollination (Jaramillo et al., 2009; Junqueira et al., 2013). Therefore, the usage of semiochemicals represents a viable alternative for controlling thrips in yellow passion fruit crops. Semiochemicals as kairomones (e.g., p-anisaldehyde) are usually regarded as long-lasting, species-specific (i.e., not harmful for natural enemies), innocuous (i.e., safe for the environment) and cost-effective (Kirk et al., 2021; Sampson and Kirk, 2013).
The kairomone p-anisaldehyde (also known as 4-methoxybenzaldehyde) is an organic compound that belongs to the class of benzaldehydes. Some studies have reported that p-anisaldehyde can be toxic to several arthropod species, including Aedes albopictus (Skuse) (Hao et al., 2014), Dermatophagoides spp. (Neri et al., 2006), Haematobia irritans irritans (L.) (Showler and Harlien, 2018), and Lycoriella ingenua (Dufour) (Park et al., 2006). Nevertheless, this kairomone was proven to be useful to increase the attractiveness of traps against the thrip species Frankliniella intonsa (Trybom), F. occidentalis (Pergande), F. tritici (Fitch), Limothrips cerealium (Haliday), Thrips fuscipennis Haliday, T. hawaiiensis (Morgan), T. imaginis Bagnall, T. major Uzel, T. obscuratus (J. C. Crawford), T. pillichi Priesner, T. tabaci Lindeman, T. vulgatissimus Haliday (see a review in Koschier 2008; Kirk et al. 2021).
To the best of our knowledge, there are no formal studies that tested the attractiveness of p-anisaldehyde to N. signifier. In this study, we assessed the effect of adding the kairomone p-anisaldehyde to sticky traps for the control of the thrip N. signifer in passion fruit crops in field conditions. Additionally, we evaluated the effect of trap height on the capture efficiency of kairomone-containing sticky traps to increase its effectiveness in passion-fruit crops.
Materials and Methods
Study site and experimental setup
The experiment was conducted at the Centro Universitario Regional Del Norte - Universidad del Tolima (CURDN) located in the municipality of Armero-Guayabal (4°59’45” N, 74°54’23” W, Tolima, Colombia). This locality lies at 300 m in flat terrain, has 28°C mean annual temperature, 1600 mm mean annual precipitation, and 71% relative humidity. We established a 50 x 68 m plot within a 3400 m2 passionfruit production area with 2 m between rows and 3 m between-plants distances. The entire crop received biweekly applications of biological insecticides (in a rotative manner) for pest control: spinetoram (spinosyn J + spinosyn L), entomopathogenic microorganisms (Beauveria bassiana, Lecanicillium lecanii and Bacillus thuringiensis), and plant extracts (Allium sativum and Capsicum spp.).
We installed 30 sticky traps in the experimental plot at 1 m height; 15 traps containing the kairomone p-anisaldehyde, and the remaining 15 traps were regarded as the control treatment. Two to three traps were placed in every-other row spanning 23 rows, and the traps within a single row were separated by 20 m. The traps that belong to the two different experimental treatments were randomly distributed within the plot (trap arrangement is shown in Fig. S1). All sticky traps were made with 20 x 25 cm straw cardboards wrapped with blue adhesive paper (490 nm) as the blue color appears to be more attractive to thrips (Brødsgaard, 1989; Kirk et al., 2021; Natwick et al., 2007; Pobozniak et al., 2020). Both faces of the trap were impregnated with gear lubricant as an insect-immobilizing medium (Valvulina SAE 140 API GL-1, Lubry, Colombia). The kairomone-containing traps were modified by cutting open a 2.5 x 5 cm window in the center of the cardboard to fit the sachet of Trip-Lure FG (Avgust Colombia S.A.S., Colombia) with 1.035 mg of p-anisaldehyde (active ingredient) and 1.08 g of inert material. The collecting permit for this study was granted by the Autoridad Nacional de Licencias Ambientales (ANLA) - resolution No. 02191 (November 27 of 2018).
Kairomone attraction assessment
All traps were monitored weekly for four months (November 2019 - February 2020), and samples for 15 time points were obtained. Every trap was inspected under a stereomicroscope (SMZ445, Nikon, Tokyo), and thrip number was recorded (adults and nymphs combined). After every monitoring event, all traps were washed, and the insect-immobilizing medium was renewed. The kairomone was replaced every three weeks (according to the manufacturer, the activity of the kairomone in field conditions may last from four to six weeks).
Thrip incidence
We sampled thrips in the terminal branches of the passion-fruit plants the same day that sticky traps were collected. Therefore, 15 sampling events in the plant adjacent to every sticky trap were carried out. The sampling of every plant was performed by beating three times one branch against a white letter paper (22x28 cm) as described by Santos et al. (2012a). Three sub-samples (i.e. sampling of one branch) were collected in every plant, and the data were pooled.
Trap-height effect
We ran an additional field assay in order to assess if the effectiveness of the sticky traps baited with the kairomone p-anisaldehyde was dependant on trap height. In this case, we installed sticky traps (trap format and spacing similar as described above) following a two-factor experiment. Traps with and without p-anisaldehyde were placed at 1, 1.6 or 2 m. Each experimental treatment was replicated five times (N=30), and the traps were inspected for five weeks. Kairomone replacement and weekly renovation of the insect-immobilizing medium was conducted as described above.
Insect identification
The thrips collected using the sticky traps and sampling directly in the passion-fruit plants were identified as N. signifer using the taxonomic keys by Mound (1996) and Lima and Mound (2016). Additionally, representative specimens were sent to a consultant (Bioquiality Agro Pa. Sas., Colombia) to confirm the taxonomic identification.
Data analysis
All statistical analyses were performed in R 4.0.2 (R Development Core Team, 2019) using RStudio (Posit Software, 2020). Insect counts from all assays were analyzed through generalized linear mixed-effects models (Bolker et al., 2009). First, data distribution was identified as negative binomial in all three analyses using the library fitdistrplus (Delignette-Muller et al., 2019). Subsequently, the models were fitted using the function ‘glmer. nb’ from the lme4 library (Bates et al., 2015). For kairomone attraction and thrip incidence, treatment (kairomone or control) and time-point (i.e., weekly evaluation) were included as fixed effects, and trap and row number were included as random effects. On the other hand, for trap-height effect-assessment, as we intended to focus on the effect of trap height rather than in the temporal variation of the caught insects, treatment (kairomone or control) and height were the fixed effects, and time-point, and trap and row number were the random effects.
Type-II analysis of variance tables were used to assess the significance of terms in the models using the ‘Anova’ function from the car library (Fox and Weisberg, 2018). The library performance (Lüdecke et al., 2019) was used to inspect and plot the model diagnostics, and figures were made using the libraries sjPlot (Lüdecke, 2016) and ggplot2 (Wickham, 2016).
Results
Kairomone attraction
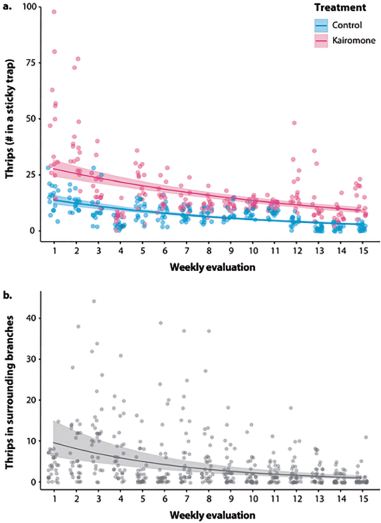
The number of collected thrips in the sticky traps was affected by both the treatment and the time-point in a two-way interaction (c2 = 5.98, df = 1, P = 0.014, see Fig. S2 for regression diagnostics). The traps baited with the kairomone p-Anisaldehyde trapped a significantly higher number of thrips than the control traps, and the number of trapped insects was reduced towards the end of the experiment (i.e., after 15 weeks) (Fig. 1a). On average, every sticky trap baited with p-anisaldehyde trapped 17.29 ± 1.82 thrips in a week, while every control trap collected 7.19 ± 0.69 thrips in the same period.
Thrip incidence
The number of thrips collected in the branches surrounding the sticky traps did not allow to differentiate whether the traps were baited with the kairomone or not. Yet, there was an effect of time-point on thrip incidence (c2 = 1035.41, df = 1, P < 0.001, see Fig. S3 for regression diagnostics). This implies that the number of thrips was significantly decreased through time (Fig. 1b). On average, 4.55 ± 0.89 thrips were collected on the surrounding branches of a trap (data from three branches pooled). However, after 15 weeks of consecutive trap activity, the number of thrips was reduced to 0.87 ± 0.84 individuals near a trap.
Source: Original from the authors.
Figure 1 Results of the kairomone attraction assessment during 15 weeks. (a). The sticky traps equipped with the kairomone p-anisaldehyde caught a significantly higher number of Neohydatothrips signifier than the control traps (i.e., without kairomone), but the number of caught insects decreased with time in both treatments (see Fig. S2 for regression diagnostics). (b) The number of thrips collected in the plants surrounding the traps was significantly reduced over time, but no statistical difference was detected between the experimental treatments (with and without kairomone) (see Fig. S3 for regression diagnostics).
Trap-height effect
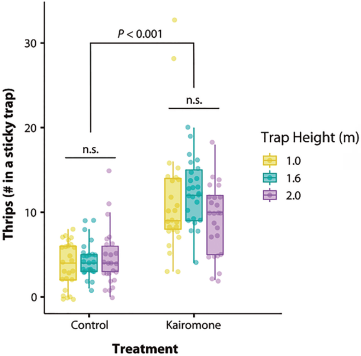
We did not detect any effect of trap height on the number of thrips collected. Nevertheless, it was again evident that the sticky traps baited with p-anisaldehyde were more effective than the control traps (c2 = 51.87, df = 1, P < 0.001, see Fig. S4 for regression diagnostics) (Fig. 2).
Source: Original from the authors.
Figure 2 The height of the trap did not affect the number of Neohydatothrips signifier caught. However, the sticky traps equipped with the kairomone consistently caught a significantly higher number of thrips (see Fig. S4 for regression diagnostics).
Discussion
This is the first study to report the effective usage of p-anisaldehyde as an attractant of N. signifier in yellow passion fruit crops. We demonstrated that equipping sticky traps with the commercially available p-anisaldehyde significantly increases the capture of this pest species in field conditions. Besides, this is the first time that the effectiveness of p-anisaldehyde has been demonstrated for a thrips species in the genus Neohydatothrips because, thus far, this compound was only used against pest thrip species belonging to the genera Frankliniella, Limothrips and Thrips (Kirk et al., 2021; Koschier, 2008).
In a first assay, we demonstrated that the addition of p-anisaldehyde increased captures of N. signifier by up to 292% in blue sticky traps (at the first census, we collected 14.93 ± 3.46 thrips/trap in the control treatment and 43.60 ± 13.29 thrips/trap in the kairomone treatment). Nevertheless, the number of caught thrips in both treatments decreased throughout the study, indicating a local reduction of the population. Considering that our experiment was conducted in yellow passion fruit crops that received the application of biological insecticides (see materials and methods section for further details), the number of N. signifier caught by the sticky traps (both with and without p-anisaldehyde) in the first weeks was considerably high. This phenomenon might indicate that insecticide application was not effective to control N. signifier, which was also pointed out by previous studies (Kirk et al., 2021; Mouden et al., 2017). Regarding the efficiency of the sticky traps, our results are in line with several previous studies comparing sticky traps with and without semiochemicals (i.e., pheromones, kairomones) which reported increases in thrip captures in the range of 20-300 % (Broughton and Harrison, 2012; Covaci et al., 2012; Sampson and Kirk, 2013).
Moreover, in a second assay, we compared the performance of blue sticky traps with and without p-anisaldehyde installed at different heights (i.e., 1, 1.6, and 2m). We found out that trap height did not affect the performance of the trap, and still, the kairomone-equipped traps caught significantly more thrips than control traps. This result implies that implementing mass-trapping campaigns using sticky traps equipped with commercially available p-anisaldehyde in real field conditions might be more accessible and more straightforward for the producers. However, although yellow passion fruit plants usually have a c.a. 2 m trellis (Jaramillo et al., 2009), it would be necessary to consider the phenological stage of the crop and install the traps in the proximity of flower buds and vegetative terminals.
While in glasshouses, the sticky traps are usually installed in a high-density arrangement (i.e., less than four meters between traps), in this study, we installed the traps more spaced apart (≥ 20 m) to avoid introducing noise in the experiment. As shown by Teulon et al. (2007), baited traps can influence the catching of nonbaited traps in a 10 m radius. Therefore, we regard that our design truly compares baited versus non-baited sticky traps, and such arrangement can be helpful for thrip population monitoring. Nevertheless, an additional study considering distances between traps should be carried out to determine the optimal arrangement for significant thrip control at the plot level. Normally, if mass trapping is intended, a higher density arrangement should be considered (Sampson and Kirk, 2013).
Considering the underlying mechanism for increased attractiveness of N. signifer to traps equipped with p-anisaldehyde, it is worth noticing that the behavioral response of thrips towards this compound had not been studied so far. Even though several semiochemicals have been successfully used to increase the collection of many thrip species, the underlying mechanism of this interaction (i.e., chemical ecology) is poorly understood. Most studies, including ours, are based on demonstrating increased catches in the field, glass-house conditions, or directional orientation in laboratory bioassays (Kirk et al., 2021). Therefore, more detailed studies are needed to understand the effects of the semiochemicals that are considered to attract thrips. Our study demonstrates that equipping blue sticky traps with the commercial kairomone p-anisaldehyde can be an effective strategy to quickly reduce the population of N. signifer in yellow passion fruit crops. In future studies, it would be interesting to test different trap arrangements and compare the effectiveness of p-anisaldehyde against other semiochemicals used for thrips control, such as ethyl nicotinate and methyl isonicotinate. Such compounds are widely used and have proven to increase the mass trapping of several thrip species (Kirk et al., 2021; Teulon et al., 2017). Additionally, assessment of thrip population size and crop damage should be ideally conducted along with the monitoring of sticky traps to provide evidence of effectiveness and economic viability of this technique as a mass-trapping method.