Introduction
Darwin wasps (Hymenoptera: Ichneumonidae) are parasitoids wasps considered one of the largest families in the animal kingdom and natural enemies of a wide range of holometabolous insects and spiders (Quicke, 2014; Klopfstein et al., 2019). According to Yu et al. (2016), there are approximately 4,420 species of Ichneumonidae in the Neotropical region, and around 25,000 species worldwide.
In Colombia, only 253 species have been recorded, which accounts for less than 1% of the global known species. However, around 80 species have been documented in recent years, indicating a gradual advancement in the study of this group in the country (Yu, et al. 2016; Bordera, et al., 2016; Herrera-Florez, 2017; Herrera-Florez, et al., 2017; Herrera-Florez, 2018; Herrera-Florez and Molina, 2018; Palacio, et al., 2018; Santos and Aguiar, 2018; Santos and Hoppe, 2018; Pádua, et al., 2019; Palacio, et al., 2019; Supeleto, et al., 2019; Araujo, et al., 2020; Pádua, et al., 2020; Supeleto, et al., 2020; Alvarado and Palacio, 2021; Claridge, 2021; Araujo, et al., 2022; Santos, et al., 2022; Supeleto and Aguiar, 2022; Pádua, et al., 2023).
One of the most significant groups within Ichneumonidae is the subfamily Cremastinae Förster, 1869. This cosmopolitan subfamily comprises 35 genera, with 14 genera known in the Neotropical region (Yu, et al., 2016).
Cremastinae plays a vital role in biological control, parasitizing a wide range of hosts, mainly caterpillars from moth families such as Coleophoridae, Hesperiidae, Psychidae, Pyralidae and Tortricidae. They also affect beetle larvae from Buprestidae and Chrysomelidae, as well as wasps of Tenthredinidae (Okada and Oike, 1940; Dasch, 1979). Additionally, some species are known to parasitize aquatic lepidopteran larvae (Gauld, 2000; Fernandes et al., 2018). There are endoparasites with a koinobiont strategy that consists of temporarily paralyzing the host and allowing it to continue growing until reaching a certain size or stage of development before causing its death (Marquina Montesinos 2019).
Distinguishing features of Cremastinae from the other Darwin wasps subfamilies include a sclerotized bridge that separates the membranous cavities of the basitarsi and tibial spurs from the tibiae (Townes, 1958).
The Cremastinae fauna from Colombia has not been extensively studied, with only three species of the EiphosomaCresson, 1865 reported by Gauld (2000) and Yu, et al., (2016). Therefore, we propose a taxonomic synopsis for the genera of Cremastinae in Caquetá, Colombia, which includes a diagnosis and an illustrated taxonomic key.
Materials and methods
This study was based on specimens collected during the field phase of the project titled “Taxonomy of Pipunculidae (Diptera: Insecta) of Colombia.” These specimens are currently deposited in the Laboratorio de Entomología de la Universidad de la Amazonia (LEUA) in Caquetá, Colombia. Darwin wasps were collected using Malaise traps and Suspendable traps continuously from October 26, 2016 to April 12, 2017, for approximately 6.5 months without interruption day and night. The sampling efforts covered both rural areas, such as sugarcane crops (Saccharum officinarum Linnaeus, 1753) from the Poaceae family, and secondary forest areas (ground and canopy) across the 16 municipalities of the Caquetá department.
For the identification and examination of the morphological characteristics, dry mounts were prepared. Direct mounting with entomological pins was used, with glue applied laterally to the mesosoma. This method prevented perforation of the specimens while preserving their morphological characters and coloration.
Individuals were examined and identified using an Olympus SZ61 stereoscope with a 2x auxiliary lens. The dichotomous keys and terminology proposed by Gauld (2000) for the genera of Cremastinae were followed. High-resolution photographs were taken at different focal depths using a Leica digital camera DFC500 attached to a Leica M205C stereomicroscope, connected to a computer with the Leica Application Suite LAS V3.6 software, which includes the Syncroscopy program to align the photographs. The images were edited using the Adobe Photoshop CS6® software tools.
The previous key by Gauld (2000) was modified to incorporate the genera of Cremastinae discovered in Caquetá, Colombia. Maps displaying the geographic records of each genus were generated using SimpleMappr software (Shorthouse, 2010).
In the list of examined specimens, label data are provided exactly as presented on the labels. Square brackets ([]) are used to indicate additional data that are not provided on the specimen labels. When specimen labels contain identical data, the term ‘idem’ is used, and only the differing data are included.
Results
A total of 306 specimens of Cremastinae were analyzed, consisting of 254 females and 52 males, representing seven genera. Gauld (2000) previously documented EiphosomaCresson, 1865 (n= 262). However, this study reveals new records for Colombia, including CreagruraTownes, 1971 (n= 1); EutanygasterCameron, 1911 (n= 2); PristomerusCurtis, 1836 (n= 1); Temelucha Förster, 1869 (n= 7); Trathala Cameron, 1899 (n= 7) and XiphosomellaSzépligeti, 1905 (n= 26) (Table 1).
Table 1 List of genera of Cremastinae from the Andean-Amazonian region, Caquetá, Colombia. New records are indicated with an asterisk (*).
| Genera | Habitat Colected (this study) | New World Distribution |
|---|---|---|
| CreagruraTownes, 1971* | Sugarcane crops (Saccharum officinarum) | Brazil, Colombia, Costa Rica, Ecuador, Suriname, Peru |
| EiphosomaCresson, 1865 | Sugarcane crops (Saccharum officinarum), and Secondary Forest (ground and canopy) | Argentina, Barbados, Brazil, Canada, Colombia, Costa Rica, Cuba, El Salvador, French Guiana, Grenada, Guatemala, Guyana, Jamaica, Mauritius é na Africa. Mexico, Paraguay, Peru, Puerto Rico, St. Vicent, Trinidad and Tobago, U.S.A, Uruguay, Venezuela |
| EutanygasterCameron, 1911* | Sugarcane crops (Saccharum officinarum) | Belize, Brazil, Colombia, Costa Rica, Mexico |
| PristomerusCurtis, 1836* | Sugarcane crops (Saccharum officinarum) | Argentina, Brazil, Colombia, Costa Rica, Honduras, Guatemala, Mexico, Nicaragua, U.S.A, Uruguay |
| TemeluchaFoerster, 1869* | Sugarcane crops (Saccharum officinarum) | Brazil, Colombia, Costa Rica, Cuba, Guatermala, Honduras, U.S.A, Uruguay |
| TrathalaCameron, 1899* | Sugarcane crops (Saccharum officinarum), and Secondary Forest (ground and canopy) | Argentina, Brazil, Colombia, Costa Rica, U.S.A, Uruguay |
| XiphosomellaSzépligeti, 1905* | Sugarcane crops (Saccharum officinarum), and Secondary Forest (ground and canopy) | Belize, Bolivia, Brazil, Colombia, Costa Rica, Grenada, Guyana, Mexico, Nicaragua, Panama, St. Vicente, Suriname, U.S.A, Uruguay |
Source: Own elaboration.
Genera of Cremastinae from Caquetá, Colombia
CreagruraTownes, 1971 (Figures 1A, 2E, 3C, 5)
CreagruraTownes, 1971: 6. Type-species: Creagrura nigripes Townes, 1971 (original designation).
Creagrura is a small Neotropical genus consisting of four species: Creagrura alejandromasisi Sääksjärvi, 2022; C. allpahuaya Sääksjärvi, 2022; C. nigripes Townes, 1971; and C. rogerblancoi Sääksjärvi, 2022. These species are distributed across Costa Rica, Brazil, Ecuador, Peru and Suriname (Townes, 1971; Sääksjärvi, et al., 2022). While some species share structural similarities with Xiphosomella, such as their slender appearance, strong thyridium, and lenticular head, the characteristic very short ovipositor sets Creagrura apart from all other New World taxa (Gauld, 2000).
Diagnosis. Coloration. The body is predominantly yellowish, with variable dorsal infusions (Fig. 1A). Head. The mandible is rotated approximately 20°, with the upper tooth facing outwards, imprinted ventrally, with a distinct ventral ridge (Fig. 3A). Mesosoma. The mesoscutum exhibits a broad, but superficially imprinted notation, while the scutellum is moderately convex with strong lateral carina (Fig. 2E). Fore wing length: ranges from 8.0 to 9.8 mm (Fig. 1A). Metasoma. The metapleuron is punctate and separated from the propodeum by a strong pleural carina. The propodeum has complete anterior and posterior carina, as well as complete lateromedial and lateral longitudinal carina between the transverse carinae. The femur lacks a ventral tooth, and the ovipositor is short and strongly curved downwards (Fig. 3C) (Gauld, 2000).
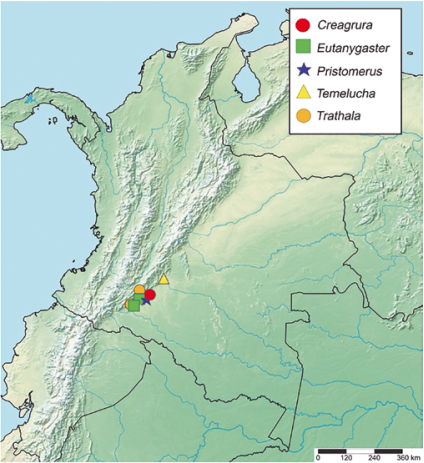
Geographical Distribution in the New World. Brazil (Acre, Amapá, Amazonas, Espírito Santo, Pará, Rio de Janeiro, Roraima); Colombia* (Caquetá) (Fig. 5); Costa Rica (Guanacaste, Heredia, Limón, Puntarenas); Ecuador (Coco); Peru (Huánuco); Suriname (Paramaribo) (Townes, 1971; Azevedo, et al., 2015; Antunes and Fernandes, 2020; Sääksjärvi, et al., 2022; Fernandes et al., 2023; Lima, et al., 2023).
Host. Lepidoptera: Hesperiidae (Sääksjärvi et al., 2022).
Examined material. 1♀. Colombia, Caquetá: El Doncello, Vereda La Arenosa, finca El Carmen, 01°40’30” N, 75°16’03” W, 322 m[metros], 07-21.XII.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA).
EiphosomaCresson, 1865 (Figures 1B, 2B, 3E, 4C, 6)
EiphosomaCresson, 1865: 52. Type-species: Eiphosoma annulatum Cresson, 1865 [=dentator Fabricius, 1804], by subsequente designation, Viereck, 1914: 50.
XiphosomaMarshall, 1892: 65. (Unjustified modification). BrachyxiphosomaViereck, 1912: 147. Type-species: Eiphosoma pyralidis Ashmead, 1896 (original designation).
Charitonedys Enderlein, 1921: 3. Type-species: Chartionedys compressum Enderlein, 1921 (original designation).
Eiphosoma is a New World genus that encompasses 56 described species, with the majority occurring in the tropical zone of South America (Gauld, 2000; Yu, et al., 2016). In Colombia, three species have been recorded: Eiphosoma laphygmae Costa Lima, 1953, Eiphosoma nigrum (Szepligeti, 1906), and Eiphosoma vitticolleCresson, 1865 (Yu, et al., 2016). Most Eiphosoma species are commonly found in lowland, open, and degraded habitats (Allen et al., 2021). Several species of Eiphosoma hold biological importance as parasitoids attacking caterpillars that are pests of Lepidoptera in agricultural systems (Pozo, 2000).
Diagnosis. Coloration. Eiphosoma species are yellow with black spots, although some species are predominantly black (Fig. 1B). Head (Fig. 4C). The mandible is not twisted and exhibits a distinct ventral edge, with the upper tooth longer and wider than the lower tooth. Mesosoma. The mesoscutum displays printed notation and punctuations concentrated in the anterior and posterior parts, while the scutellum can be smooth or punctuated. The metapleuron may be smooth or dotted, and a pleural carina is present. The propodeum can be smooth, coriaceous, rough, or striated, and carinae are present on the posterior femur with a ventral tooth. Fore wing length between 3.8 to 9.7 mm (Fig. 3E), with or without 3rs-m vein. Metasoma. The laterotergite II lacks a thyridium (Fig. 2B), and the ovipositor is either straight or possesses a sinuous apex (Gauld, 2000).
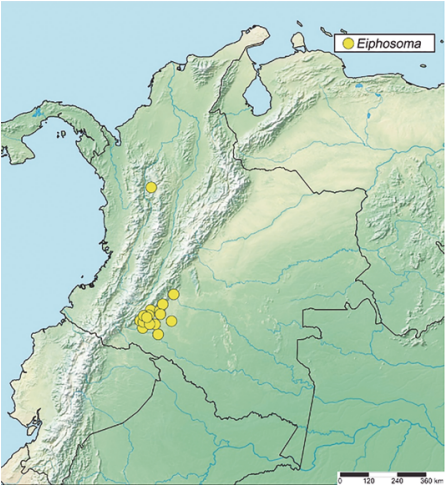
Geographical Distribution in the New World. Argentina (Buenos Aires); Barbados (Oldsbury); Belize (Cayo District); Bolivia (Cochabamba); Brazil (Amazonas, Bahia, Espírito Santo, Goiás, Maranhão, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Pará, Rio de Janeiro, Rio Grande do Norte, São Paulo); Canadá (British Columbia, Ontario, Quebec); Colombia (Caquetá, Antioquia) (Fig. 6); Costa Rica (Guanacaste, Alajuela, Puntarenas, Heredia, Limón); Cuba (Ciudad de la Habana, La Habana, Matanzas, Villa Clara, Sancti Spiritus, Camagüey, Granma, Santiago de Cuba); El Salvador (San Salvador); French Guiana; Grenada (Mount Gay); Guatemala (Alta Verapaz); Guyana (Georgetown); Haiti, Honduras (El Paraiso); Jamaica (Kingston); Mauritius (Port Louis); Mexico (Chiapas, Tamaulipas, Vera cruz); Paraguay (Asunción); Peru (Lambayeque); Puerto Rico (Moca); St. Vicent; Trinidad and Tobago (Trinidad); U.S.A (Alabama, Arizona, Arkansas, California, Colorado, Connecticut, District of Columbia, Florida, Georgia, Illinois, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Missouri, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming); Uruguay (Rocha); Venezuela (Aragua, Puerto Cabello) (Yu, et al., 2016; Azevedo, et al., 2015; Shimbori, et al., 2017; Salas-Marina, et al., 2018; Fernandes, et al., 2019a; Fernandes, et al., 2019b; Antunes and Fernandes, 2020; Fernandes, et al., 2020; Gaona-García, et al, 2020; Santos, et al., 2021; Barata, et al., 2022; Fernandes et al., 2023).
Host. Lepidoptera: Crambidae, Erebidae, Gelechiidae, Noctuidae, Pyralidae, (Yu et al., 2016).

Figure 1 Females of Darwin wasps Cremastinae from Caquetá, Colombia: A. Creagrura sp.; B. Eiphosoma sp.;C. Eutanygaster sp.; D. Pristomerus sp.; E. Temelucha sp.; F. Trathala sp.; G. Xiphosomella sp.
Examined material. Colombia, Caquetá: 213♀, 49♂. Albania, Vereda Florida 1, finca San Isidro, 01°14’50”N, 75°52’34”W,295 m[metros], 26.X.-09.XI.2016, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem 01-15.II.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♂, LEUA); idem 29.III.-12.IV.2017, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem 29.III.-12.IV.2017, (1♂, LEUA); idem (1♀, LEUA); idem 01°15’08”N, 75°53’05”W, 283 m[metros], 23.XI.-07.XII.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 15-29. III.2017, (1♀, LEUA); idem 29.III.-12.IV.2017, (1♀, LEUA); idem 29.III.-12.IV.2017, (1♀, LEUA); idem Belén de los Andaquíes, Vereda Aletones, finca La Cabaña, 01°29’31”N, 75°52’20” W, 369 m[metros], 01-15.I.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 01-15.III.2017, (1♂, LEUA); idem 15-29.III.2017, (1♂, LEUA); idem Cartagena del Chairá, Vereda Tigrera Alta, finca Las Palmeras, 01°17’5”N, 74°49’1” W, 235 m[metros], 01-15.II.2017, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Curillo, Vereda La Primavera, finca Bella Luz, 01°01’42”N, 75°54’21” W, 248 m[metros], 26.X.-09.XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. RamosPastrana (1♀, LEUA); idem (1♂, LEUA); idem 15-29.III.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♂, LEUA); idem El Doncello, Vereda La Arenosa, finca El Carmen, 01°40’30”N, 75°16’03” W, 322 m[metros], 26.X.-09. XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 01°41’06”N, 75°15’32” W, 322 m[metros], 07-21.XII.2016, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♂, LEUA); idem trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 21.XII.2016-04.I.2017, (1♀, LEUA); idem 18.I.-01.II.2017, (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem 15-29.III.2017, (1♀, LEUA); idem El Paujil, Vereda San Pedro, finca Buenos Aires, 01°32’56”N, 75°14’21” W, 283 m[metros], 15.II.-01.III.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 15-29.III.2017, (1♀, LEUA); idem (1♀, LEUA); idem Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 01-15.III.2017, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem Vereda Tarqui, 01°51’04”N, 75°40’01” W, 1719 m[metros], 15-29.III.2017, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem Milán, Vereda San Rafael, finca Bellavista, 01°09’56”N, 75°26’24” W, 231 m[metros], 23.XI.-07.XII.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 21.XII.2016-04.I.2017, (1♀, LEUA); idem 04-18.I.2017, (1♀, LEUA); idem 15.II.-01. III.2017, (1♀, LEUA); idem 15.II.-01.III.2017, (1♂, LEUA); idem 01-15.I.2017, (1♀, LEUA); idem (1♀, LEUA); idem (1♂, LEUA); idem 15-29.III.2017, (1♂, LEUA); idem 29.III.-12.IV.2017, (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♂, LEUA); idem (1♂, LEUA); idem Morelia, Vereda Albano, finca Guacari, 01°25’41”N, 75°44’56”W, 251 m[metros], 26.X.-09.XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 01-15. III.2017, (1♀, LEUA); idem Puerto Rico, Vereda La Soledad, finca Borinquen, 01°55’26”N, 75°08’58” W, 270 m[metros], 09-23.XI.2016, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem 01°55’30”N, 75°08’33” W, 308 m[metros], 23.XII.-07.XII.2016, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♂, LEUA); idem 01-15.III.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem San José del Fragua, Vereda Bellavista, finca Mi Ranchito, 01°18’23”N, 76°00’32” W, 265 m[metros], 11-23. IX.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 03-15.III.2017, (1♂, LEUA); idem San Vicente del Caguán, Vereda Alto Quebradón, finca Veracruz, 02°17’52”N, 74°44’15” W, 374 m[metros], 26.X.-09. XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem (1♂, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem 09-23. XI.2016, (1♀, LEUA); idem 07-21.XII.2016, (1♀, LEUA); idem (1♀, LEUA); idem 18.I.-01.II.2017, (1♀, LEUA); idem¸(1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem 01-15.II.2017, (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♂, LEUA); idem¸(1♂, LEUA); idem 15.II.-01.III.2017, (1♀, LEUA); idem (1♀, LEUA); idem (1♂, LEUA); idem Solano, Vereda Las Mercedes, finca La Ceiba, 0°47’07”N, 75°19’30” W, 211 m[metros], 26.X.-09.XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 09-23.XI.2016, (1♀, LEUA); idem (1♀, LEUA); idem 07-21.XII.2016, (1♂, LEUA); idem 18.I.-01.II.2017, (1♀, LEUA); idem trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♂, LEUA); idem 01-15.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♂, LEUA); idem 28.III.-12.IV.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♂, LEUA); idem Solita, Vereda Campo Lejano, finca Los Pinos, 0°57’59”N, 75°37’48”W, 233 m[metros], 18.I.-01.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Valparaíso, Vereda La Florida, finca La Florida, 01°10’18”N, 75°38’40” W, 235 m[metros], 23.XI.-07.XII.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 07-21.XII.2016, (1♀, LEUA); idem 04-18.I.2017, (1♂, LEUA); idem (1♂, LEUA); idem Solano, Vereda Las Mercedes, finca La Ceiba, 0°47’07”N, 75°19’30” W, 211 m[metros], 21.XII.2016-04.I.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem El Doncello, Vereda La Arenosa, finca El Carmen, 01°40’30”N, 75°16’03” W, 322 m[metros], 15-29.III.2017, trampa Malaise en bosque secundario - suelo, Y. RamosPastrana (1♀, LEUA); idem 15.II.-01.III.107, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem (1♂, LEUA); idem Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 21.XII.2016-04.I.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♂, LEUA); idem Vereda Tarqui, 01°51’04”N, 75°40’01” W, 1719 m[metros], 15.II.-01. III.2017, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♂, LEUA), idem (1♂, LEUA); idem Vereda La Viciosa, CIMAZ Macagual, 01°29’55”N, 75°39’25” W, 249 m[metros], 18.I.-01.II.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem Curillo, Vereda La Primavera, finca Bella Luz, 01°01’42”N, 75°54’21” W, 248 m[metros], 08-23.XI.2016, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda Paraíso, finca Paraíso, 01°44’47”N, 75°37’40”W, 716 m[metros], 18.I.-01.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem San Vicente del Caguán, Vereda Alto Quebradón, finca Veracruz, 02°17’52”N, 74°44’15” W, 314 m[metros], 15-29.III.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀,LEUA); idem 01-15.II.2017 (1♀, LEUA); idem 09-23.XI.2016 (1♀, LEUA), idem 26.X.-09.XI.2016 (1♀,LEUA); idem Albania, Vereda Florida 1, finca San Isidro, 01°14’50”N, 75°52’34”W, 295 m[metros], 04-18.I.2017 , trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 26. IX.-09.X.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. RamosPastrana (1♀,LEUA); idem San Vicente del Caguán, Vereda Alto Quebradón, finca Veracruz, 02°17’52”N, 74°44’15” W, 374 m[metros], 18.I.-01.II.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀,LEUA); idem Cartagena del Chairá, Vereda Tigrera Alta, finca Las Palmeras, 01°17’5”N, 74°49’1” W, 235 m[metros], 04-18.I.2017, trampa Malaise en bosque secundario - dosel, Y. RamosPastrana (1♀, LEUA); idem Valparaíso, Vereda La Florida, finca La Florida, 01°10’18”N, 75°38’40” W, 235 m[metros], 29.III.-04.IV.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 09-23. XI.2016, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♂, LEUA); idem San Vicente del Caguán, Vereda Alto Quebradón, finca Veracruz, 02°17’52”N, 74°44’15” W, 374 m[metros], 15.II.-01.III.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem 23.XI.- 07.XII.2016 (1♀, LEUA); idem Valparaíso, Vereda La Florida, finca La Florida, 01°10’18”N, 75°38’40” W, 235 m[metros], 26.X.-09.XI.2016, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem El Doncello, Vereda La Arenosa, finca El Carmen, 01°40’30”N, 75°16’03” W, 322 m[metros], 15-29.III.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 18.I.-01.II.2017, (1♀, LEUA); idem 23.XI.-07. XII.2016, (1♀, LEUA); Albania, Vereda Florida 1, finca San Isidro, 01°14’50”N, 75°52’34”W, 295, 04-14.i.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem 01°15’08”N, 75°53’05”W, 283 m[metros], 01-15.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 15-29.III.2017, (1♀, LEUA); idem Belén de los Andaquíes, Vereda Aletones, finca La Cabaña, 01°29’31”N, 75°52’20” W, 369 m[metros], 15.II.-01.III.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem 07-21.XII.2016. trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); Curillo, Vereda La Primavera, finca Bella Luz, 01°01’42”N, 75°54’21” W, 248 m[metros], 26.X.-09.XI.2016, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda La Viciosa, CIMAZ Macagual, 01°29’55”N, 75°39’25” W, 249 m[metros], 18.I.-01.II.2017, trampa Malaise en bosque secundario - suelo, Y. RamosPastrana (1♂, LEUA); idem (1♂, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem 01°30’32”N, 75°40’28” W, 253 m[metros], 18.I.-01.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem (1♀, LEUA); idem 23.XI.-07.XII.2016, (1♂, LEUA); idem (1♂, LEUA): idem 04-28.i.2017, (1♀, LEUA); idem 15.II-01.III.2017, (1♀, LEUA); idem Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 18.I.-01.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♂, LEUA); idem Milán, Vereda San Rafael, finca Bellavista, 01°09’56”N, 75°26’24” W, 231 m[metros], 07-21.XII.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. RamosPastrana (1♂, LEUA); idem; (1♂, LEUA); idem San José del Fragua, Vereda Bellavista, finca Mi Ranchito, 01°18’23”N, 76°00’32” W. altitud: 265 m[metros], 26.X.-09. XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 18.I.-01.II.2017, (1♀, LEUA); idem 01-15.II.2017, (1♀, LEUA); idem Solano, Vereda Las Mercedes, finca La Ceiba, 0°47’07”N, 75°19’30” W, 211 m[metros], 26.X.-09.XI.2016, trampa Malaise en bosque secundario - suelo, Y. RamosPastrana (1♀, LEUA); idem (1♀, LEUA); idem 15.II.-01.III.2017, (1♀, LEUA); Valparaíso, Vereda La Florida, finca La Florida, 01°10’18”N, 75°38’40” W, 235 m[metros], 04-18.I.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 26.X.-09.XI.2016, (1♀, LEUA); idem Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 04-18.I.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem San Vicente del Caguán, Vereda Alto Quebradón, finca Veracruz, 02°17’52”N, 74°44’15” W, 374 m[metros], 26.X.-09.XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda La Viciosa, CIMAZ Macagual, 01°30’32”N, 75°40’28” W, 253 m[metros], 26.X.-09.XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 15.II.-01.III.2017, (1♀, LEUA); idem 04- 18.I.2017, (1♀, LEUA); idem Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♂, LEUA), idem 26.X.-09.XI.2016, trampa Malaise em cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 04- 18.i.2017, (1♀, LEUA); idem Albania, Vereda Florida 1, finca El Jardín, 01°15’08”N, 75°53’05”W, 283 m[metros], 26.X.-09.XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 04-18.I.2017, (1♂, LEUA); idem 15.II.-01.III.2017, (1♀, LEUA); idem Solano, Vereda Las Mercedes, finca La Ceiba, 0°47’07”N, 75°19’30” W, 211 m[metros], 01-18.IV.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 07-21.XII.2016, (1♀, LEUA); idem Solita, Vereda Campo Lejano, finca Los Pinos, 0°57’59”N, 75°37’48”W, 233 m[metros], 18.I.-01.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem San Vicente del Caguán, Vereda Alto Quebradón, finca Veracruz, 02°17’52”N, 74°44’15” W, 374 m[metros], 18.I.-01.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem La Montañita, Vereda Morros, finca Las Dalias, 01°29’24”N, 75°24’09”W, 348 m[metros], trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♂, LEUA), idem Belén de los Andaquíes, Vereda Aletones, finca La Cabaña, 01°29’31”N, 75°52’20” W, 369 m[metros], trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Cartagena del Chairá, Vereda Tigrera Alta, finca Las Palmeras, 01°17’5”N, 74°49’1” W, 235 m[metros], trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♂, LEUA); idem Florencia, Vereda La Viciosa, CIMAZ Macagual, 01°15’08”N, 75°53’05”W, 283 m[metros], 26.X.-09.XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda La Viciosa, CIMAZ Macagual, 01°15’08”N, 75°53’05”W, 253 m[metros], 26.X.-09. XI.2016, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem Milán, Vereda San Rafael, finca Bellavista, 01°09’56”N, 75°26’24” W, 231 m[metros], 29.III.-12.IV.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda La Viciosa, CIMAZ Macagual, 01°15’08”N, 75°53’05”W, 253 m[metros], 23.XI.-07.XII.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA), idem Milán, Vereda San Rafael, finca Bellavista, 01°09’56”N, 75°26’24” W, 231 m[metros], 07-21.XII.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. RamosPastrana (1♀, LEUA); idem 04-18.I.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 26.X.-09.XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 04-18.I.2017, (1♀, LEUA); idem 26.IX.-09.X.2016 (1♀, LEUA); idem Albania, Vereda Florida 1, finca El Jardín, 01°15’08”N, 75°53’05”W, 283 m[metros], 01-15. II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643m[metros], 07-21.XII.2016, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem 07-21.XII.2016, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem El Doncello, Vereda La Arenosa, finca El Carmen, 01°41’06”N, 75°15’32” W, 322 m[metros], 23.XI.-07.XII.2016, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem 07-21. XII.2016, (1♀, LEUA); idem (1♀, LEUA); idem 21.XII.2016-04.I.2017, (1♀, LEUA); idem trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♂, LEUA); Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 01-15.III.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♂, LEUA); idem Solano, Vereda Las Mercedes, finca La Ceiba, 0°47’07”N, 75°19’30” W, 211 m[metros], 18.I.-01.II.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem Solano, Vereda Las Mercedes, finca La Ceiba, 0°47’07”N, 75°19’30” W, 211 m[metros], 18.I.-01.II.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem 15-29.III.-2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Cartagena del Chairá, Vereda Tigrera Alta, finca Las Palmeras, 01°17’5”N, 74°49’1” W, 235 m[metros], 04-18.I.2017, trampa Malaise en bosque secundario - dosel, Y. RamosPastrana (1♀, LEUA); idem Solita, Vereda Campo Lejano, finca Los Pinos, 0°57’59”N, 75°37’48”W, 233 m[metros], 07-21.XII.2016, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem El Doncello, Vereda La Arenosa, finca El Carmen, 01°41’06”N, 75°15’32” W, 322 m[metros], 07-21.XII.2016, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem Valparaíso, Vereda La Florida, finca La Florida, 01°10’18”N, 75°38’40” W, 235 m[metros], 04-18.I.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem Solano, Vereda Las Mercedes, finca La Ceiba, 0°47’07”N, 75°19’30” W, 211 m[metros], 01-15.II.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. RamosPastrana (1♀, LEUA); idem Cartagena del Chairá, Vereda Tigrera Alta, finca Las Palmeras, 01°17’5”N, 74°49’1” W, 235 m[metros], 01-15.II.2017, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♂, LEUA); Idem trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem 21.XII.2016-04.I.2017, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem Milán, Vereda San Rafael, finca Bellavista, 01°09’56”N, 75°26’24” W, 231 m[metros], 04-18.I.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Solano, Vereda Las Mercedes, finca La Ceiba, 0°47’07”N, 75°19’30” W, 211 m[metros], 01-15.I.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem San Vicente del Caguán, Vereda Alto Quebradón, finca Veracruz, 374 m[metros], 23.XI.-07.XII.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem San Vicente del Caguán, Vereda Alto Quebradón, finca Veracruz, 02°17’52”N, 74°44’15” W, 374 m[metros], 23.XI.-07.XII.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. RamosPastrana (1♀, LEUA); idem San José del Fragua, Vereda Bellavista, finca Mi Ranchito, 01°18’23”N, 76°00’32” W, 265 m[metros], 23.XI.-07.XII.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 09-23.XI.2016, (1♀, LEUA); idem Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 04-18.I.2018, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem 15.II.-01.III.2017, (1♀, LEUA).
EutanygasterCameron, 1911 (Figures 1C, 3F, 4D, 5)
EutanygasterCameron, 1911: 182. Type-species: Eutanygaster brevipennis Cameron (monotypy).
Eutanygaster is a small Neotropical genus with two described species: Eutanygaster brevipennis Cameron, 1911 and Eutanygaster tabascensis (Morley, 1912). Additionally, there are several undescribed taxa within this genus (Gauld, 2000). In the past, Eutanygaster was often confused with Xiphosomella; and the species E. tabascensis was incorrectly placed within that genus (Townes and Townes, 1966).
Diagnosis. Coloration. Primarily black, although some specimens may exhibit a more yellowish ventral coloration (Fig. 1C). The head (Fig. 4D) is characterized by short mandibles that are not crooked, with a prominent ventral ridge. The upper tooth is slightly longer and thicker than the lower tooth. In the mesosoma, the mesoscutum has a broad, but superficially imprinted notation, appearing smooth and punctuated. The scutellum is convex and smooth, lacking lateral carinae. Abbreviations: T: Thyridium; Lt: Laterotergite; Clle: Scutellar lateral longitudinal carina. carinae; Mesopleuron smooth, dotted anteriorly; propodeum with complete anterior and posterior carinae. Fore wing 4.5 to 6.0 mm (Fig. 1C, 3F). It lacks an areolet, but has contiguous M and Rs veins, 2rs-m is absent. The pterostigma is very thin. In the metasoma, tergite II lacks a thyridium and has a pendant laterotergite (Gauld, 2000).
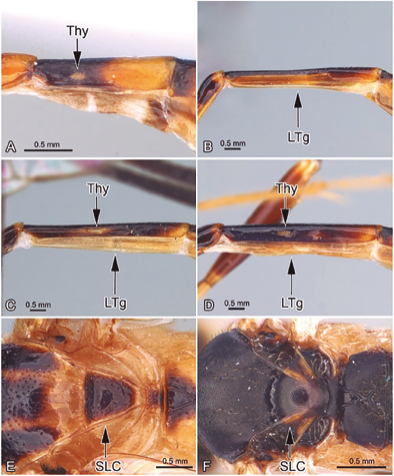
Figure 2 A. Pristomerus sp. Tergite II, lateral view; B. Eiphosoma sp. Tergite II, lateral view; C. Creagrura sp. Tergite II, lateral view; D. Xiphosomella sp. Tergite II, lateral view; E. Creagrura sp. Scutellum; F. Xiphosomella sp., Scutellum.
Geographical Distribution in the New World. Belize (Cayo District); Brazil (Amazonas, Espírito Santo, São Paulo); Colombia* (Caquetá) (Fig. 5).; Costa Rica (Guanacaste, Heredia, Puntarenas, San Jose); Guyana; Mexico (Tabasco) (Yu et al., 2016; Azevedo et al., 2015; Antunes & Fernandes, 2020; Fernandes et al., 2023).
Host. Lepidoptera: Gelechiidae (Reis et al., 2010).
Examined material. 2♀. Colombia, Caquetá: Florencia, Vereda La Viciosa, CIMAZ Macagual, 01°30’32”N, 75°40’28” W, 253 m[metros], 04-18.I.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem Albania, Vereda Florida 1, finca El Jardín, 01°15’08”N, 75°53’05”W, 283 m[metros], 21.XII.2016-04.IV.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA).
PristomerusCurtis, 1836 (Figures 1C, D, 5)
PristomerusCurtis, 1836: 624. Type-species: Ichneumon vulneratorPanzer, 1799 (original designation).
PristomerideaAshmead, 1900: 100. Type-species: Porizon agilis Cresson, 1872 [= autrinus Townes, 1951] (original designation).
PristocelusSzépligeti, 1905: 48. Type-species: Pristocelus atriceps Szépligeti, 1905 (monotypy). NeopristomerusViereck, 1912: 592. Type-species: Pristomerus appalachianusViereck, 1905 [Ophion spinator Fabricius, 1804] (original designation).
Nesanomalon Morley, 1913: 56. Type-species: Nesanomalon dimidiatum Morley, 1913 (monotypy).
Pristomerus is a cosmopolitan genus that includes 99 registered species (Yu et al., 2016), most distributed in tropical and subtropical areas. Larvae are endoparasitic of Lepidoptera. In the New World tropics, Pristomerus is not as species-rich as in the Old World, perhaps because the closely related endemic genus Xiphosomella is extremely species-rich (Gauld, 2000).
Diagnosis. Coloration is mainly yellowish to reddish brown (Fig. 1D). The head (Fig. 1C) features untwisted mandible, without a prominent ventral border, with slightly biconcave upper tooth. The mesosoma has a moderately to weakly imprinted notation on the mesoscutum, which can be smooth or granular with few punctures. The scutellum is moderately convex, smooth and polished without lateral carinae. The metapleuron is usually dotted, sometimes granular or leathery, and the propodeum usually has complete anterior and posterior carinae. The fore wings measure 2.1 to 5.1 mm. (Fig. 1D) (Gauld, 2000).
Geographical Distribution in the New World. Argentina; Brazil (Amazonas, Espírito Santo, Mato Grosso do Sul, São Paulo); Colombia* (Caquetá) (Fig. 5); Costa Rica (Guanacaste, Heredia, Limón, Puntarenas, San Jose); Honduras; Guatemala; Mexico; Nicaragua (La Calera); U.S.A (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, Washington, West Virginia, Wisconsin, Wyoming); Uruguay (Rocha) (Yu et al., 2016; Azevedo et al., 2015; Shimbori et al., 2017; Fernandes et al., 2019b; Fernandes et al., 2020; Santos et al., 2021; Fernandes et al., 2023).
Host. Diptera: Anthomyiidae; Coleoptera: Curculionidae; Hymenoptera: Braconidae, Cynipidae, Tenthredinidae; Lepidoptera: Agonoxenidae, Alucitidae, Blastobasidae, Carposinidae, Coleophoridae, Cosmopterigidae, Cossidae, Depressariidae, Gelechiidae, Geometridae, Hyblaeidae, Lasiocampidae, Lycaenidae, Lymantriidae, Noctuidae, Nolidae, Nymphalidae, Pieridae, Plutellidae, Pyralidae, Scythridae, Sesiidae, Tortricidae, Yponomeutidae, Zygaenidae (Gauld, 2000; Domínguez-Jiménez, 2000; Yu et al., 2016).
Examined material. 1♀. Colombia, Caquetá: La Montañita, Vereda Morros, finca Las Dalias, 01°29’21.5”N, 75°24’17”W, 290 m[metros], 15.II.-01.III.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA).
Temelucha Forester, 1869 (Figures 1E, 4E, 5)
TemeluchaFoerster, 1869: 148. Type-species: Porizon macer Cresson, 1872 [Porizon facilis Cresson, 1872] by subsequent designation [Perkins, 1962: 457.]
Temelucha is a widespread genus found across the globe, with 232 described species and numerous undescribed species, particularly in the hotter, drier regions of the Old World and Australia (Yu et al., 2016). Interestingly, the number of species found in cool and moist habitats such as the British Isles is relatively low (Fitton and Gauld, 1980). Surprisingly, Temelucha seems to be sparsely represented in many parts of tropical America.
Temelucha is easily distinguished from other Cremastinae genera by the shape of the first metasoma segment, where the tergite margins enclose the sternites. Temelucha species do not have a tooth on the posterior femur or a discernible thyridium.
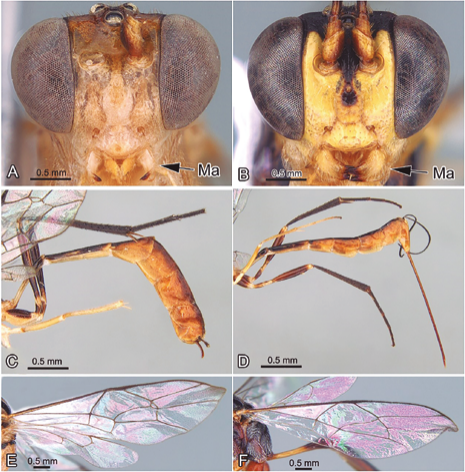
Figure 3 A. Creagrura sp. Head; B. Xiphosomella sp. Head; C. Creagrura sp. Ovipositor; D. Xiphosomella sp. Ovipositor; E. Eiphosoma sp. Fore wing; F. Eutanygaster sp. Fore wing. Abbreviations: Ma: Mandible.
Only a few species have a sinuous apex on the ovipositor. Morphologically, they bear resemblance to Trathala species, although Temelucha species never have the first tergite enclosing sternites I. Additionally, Temelucha species closely resemble Neleothymus Förster, 1869 species, suggesting that Neleothymus could be considered as a specialized group within Temelucha (Gauld, 2000).
Diagnosis. Coloration. Mainly yellowish-brown with few black markings (Fig. 1E). Head. (Fig. 1E). The mandible is not twisted with a narrow ventral ridge, with the upper tooth slightly longer and sturdier than the lower tooth. Mesosoma: The mesoscutum exhibits weakly imprinted or vestigial notation, generally appearing granulated with scattered punctures, while the scutellum is typically flattened. The mesopleuron is generally smoothed with fine punctures anteriorly. The propodeum usually displays more or less complete carinae, although the superomedial area and petiolar can be confluent, sometimes with a transversely strigose area. Fore wing length ranges from 2.2 to 4.5 mm (Fig. 1E). Metasoma. The first segment of the metasoma features tergite margins enclosing the sternites (Fig. 4E). No species within the genus possess a tooth on the posterior femur, and the thyridium is not detectable. Only a few species have a sinuous apex on the ovipositor (Gauld, 2000).
Geographical Distribution in the New World. Brazil (Bahia, Espírito Santo, Mato Grosso do Sul, São Paulo); Colombia* (Caquetá) (Fig. 5); Costa Rica (Cartago, Guanacaste, Puntarenas); Cuba; Guatemala; Honduras; U.S.A (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, Wyoming); Uruguay (Rocha) (Yu et al., 2016; Azevedo et al., 2015; Fernandes et al., 2019a; Fernandes et al., 2019b; Fernandes et al., 2020; Santos et al., 2021).
Host. Lepidoptera: Gelechiidae, Noctuidae Pyralidae, Tortricidae (Gauld, 2000; Yu et al., 2016; Vas, 2016).
Examined material. 7♀. Colombia, Caquetá: San Vicente del Caguán, Vereda Alto Quebradón, finca Veracruz, 02°17’52”N, 74°44’15”W, 374 m[metros], 26.X.-09. XI.2016, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. RamosPastrana (1♀, LEUA); idem (1♀, LEUA); idem 21.XII.2016-04.I.2017, (1♀, LEUA); idem 09-23.XI.2016 (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem 18.I.-01.II.2017, (1♀, LEUA).
TrathalaCameron, 1899 (Figures 1F, 4F, 5)
TrathalaCameron, 1899: 122. Type-species: Trathala striata Cameron, 1899 (monotypy).
EpicremastusSzépligeti, 1905: 51. Type-species: Epicremastus concolor Szépligeti, 1905 (monotypy).
PaurolexisCameron, 1907: 587. Type-species: Paurolexis flavus Cameron, 1905 (monotypy).
HaristaeusCameron, 1909 [1910]: 442. Type-species: Haristaeus nigrifrons Cameron, 1909 (monotypy).
Trathala is a cosmopolitan genus comprising 97 described species (Yu et al., 2016). Structurally, Trathala exhibits a moderate range of variations in both, the Old and New Worlds, and its differentiation from the Holarctic genus Cremastus Gravenhorst, 1829 and the South African genus RicrenaCameron, 1906 is not entirely clear. These issues, along with the classification status of the genus, can only be resolved when the highly diverse tropical faunas are better understood in terms of natural species groups within the Ichneumonidae (Gauld, 2000).
Diagnosis. Coloration. The coloration is variable, with complete blackness being rare (Fig. 1F). Head. (Fig. 1F) The mandibles are not twisted and lack a prominent ventral flange. Generally, the upper tooth is slightly longer and stouter than the lower tooth, although there are cases where the lower tooth is slightly longer. Mesosoma. The mesoscutum exhibits weak to moderately strong notauli impressions, and it is typically granulated or punctuated with granular sculpture on interstices. The scutellum is convex and exhibits various sculptures, usually lacking carinae laterally, epicnemial carina is complete, and the posterior transverse carina is complete or slightly raised medioventrally. The propodeum features anterior and posterior transverse carinae, generally complete, with the presence of lateromedial and laterolongitudinal carinae often complete between the transverse carinae, rarely with both absent. The fore wing lacks an enclosed areolet, and the pterostigma is moderately stout, usually as wide or wider than the first subdiscal cell. The fore wing length ranges from 1.7 to 11.8 mm (Fig. 1F). Metasoma. Tergite II without thyridium, and a folded laterotergite is present beneath the under tergite. The margins of tergite I are parallel and widely separated, thereby exposing the sternites (Fig. 4F). Notably, no Trathala species possesses an areolet on the fore wing, and they also lack a tooth on the posterior femur (Gauld, 2000).
Geographical Distribution in the New World. Argentina (Mendoza); Bolivia; Brazil (Amazonas, Bahia, Espírito Santo, Rio Grande do Norte, Santa Catarina); Colombia* (Caquetá) (Fig. 5); Costa Rica (Guanacaste, Heredia, Limón, Puntarenas, San José); U.S.A (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Vermont, Virginia, West Virginia, Wisconsin); Uruguay (Rocha) (Yu et al., 2016; Azevedo et al., 2015; Fernandes et al., 2019a; Fernandes et al., 2019b; Fernandes et al., 2020a; Barata, et al., 2022; Fernandes et al., 2023).
Host. Coleoptera: Cerambycidae, Chrysomelidae, Cleridae, Curculionidae, Trogossitidae; Hymenoptera: Cynipidae, Tenthredinidae; Lepidoptera: Arctiidae, Batrachedridae, Carposinidae, Cosmopterigidae, Depressariidae, Gelechiidae, Gracillariidae, Lasiocampidae, Noctuidae, Oecophoridae, Pyralidae, Tineidae, Tortricidae (Yu, et al., 2016).
Examined material. 5♀. Colombia, Caquetá: Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°2’24”N, 75°36’36”W, 643 m[metros], 01-15.II.2017, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem Vereda Tarqui, 01°51’04”N, 75°’40’01”W, 1719 m[metros], 01-15.II.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem El Doncello, Vereda La Arenosa, finca El Carmen, 01°40’30”N, 75°16’03” W, 322 m[metros], 15.II.-01.III.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem San José del Fragua, Vereda Bellavista, finca Mi Ranchito, 01°18’23”N, 76°00’32”W, 265 m[metros], 07-21. XII.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA, idem Albania, Vereda Florida 1, finca El Jardín, 01°15’08”N, 75°53’05”W, 283 m[metros], 15-29.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA).
XiphosomellaSzépligeti, 1905 (Figures 1G, 2F, 3B, 7)
XiphosomellaSzépligeti, 1905: 4. Type-species: Xiphosomella brasilensis Szépligeti, 1905 (monotypy).
AreolopristomerusCushman, 1920: 274. Type-species: Pristomerus (Areolopristomerus) smithi Cushman, 1920 [= dubia Brues] (original designation).
Xiphosomella is a heterogeneous genus found in the New World, comprising 54 described species (Yu, et al., 2016). Additionally, a significant number of species are still awaiting description. In terms of structure, Xiphosomella, as currently defined Townes (1971), exhibits the widest range of variation among all the genera in the Cremastinae subfamily. This stands in contrast to other parts of the Cremastinae, where the generic distinction is based on morphologically distinct species groups (Gauld, 2000). This situation has been further complicated by the fact that the difference between Xiphosomella and Pristomerus is not as straightforward as suggested by Townes (1971). Presently, the primary distinguishing feature between the two genera is the position of the thyridium. In Xiphosomella, the thyridium can be located close to the anterior margin of tergite II to approximately in the center of the tergite. In Pristomerus, the thyridium is consistently found near the anterior margin of the tergite (Gauld, 2000).
Diagnosis. Coloration. Xiphosomella species exhibit a predominantly yellowish or reddish-brown coloration, often with varying infusions. However, there are rare cases where the coloration is mostly black, although this is not common (Fig. 1G). Head. The mandible is not crooked and lacks a prominent ventral ridge. Typically, the upper tooth is slightly longer, and the lower tooth is more robust, although there are rare instances where the lower tooth is longer (Fig. 3B). Mesosoma. The mesoscutum shows a range of notation impressions, varying from weak to strongly imprinted. It is usually smooth or grainy with scattered punctures, although extensive punctuation is rare. The fore wings measure 2.4 to 8.3 mm (Fig. 1G). The scutellum is moderately convex, smooth and polished without lateral carinae (Fig. 2F). The mesopleuron is generally smooth, with fine punctuations in the anterior part. The propodeum typically has both anterior and posterior transverse carinae, which are usually complete, although there are cases where both carinae are absent (Gauld, 2000).
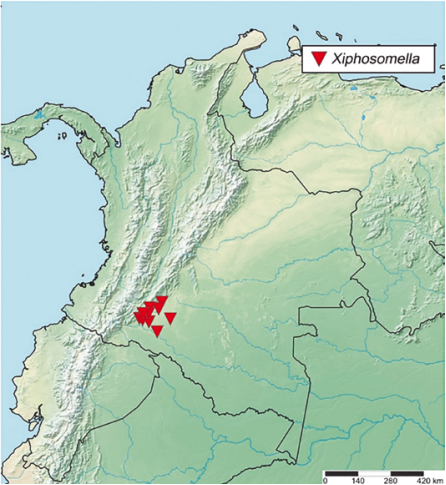
Geographical Distribution in the New World. Belize; Bolivia (Mapiri); Brazil (Amazonas, Bahia, Espírito Santo, Pará, São Paulo, Santa Catarina, Mato Grosso do Sul, Rio Grande do Norte); Canada (Ontario); Colombia* (Caquetá) (Fig. 7); Costa Rica (Cartago, Guanacaste, Heredia, Limón, Puntarenas); Grenada; Guyana; Mexico; Nicaragua; Panama (Gamboa); St. Vicente (Petit Bordel); Suriname; U.S.A; Uruguay (Rocha) (Yu, et al., 2016; Azevedo, et al., 2015; Fernandes, et al., 2019a; Fernandes et al., 2019b; Antunes and Fernandes, 2020; Fernandes, et al., 2020a; Fernandes, et al., 2020b; Santos, et al., 2021; Barata, et al., 2022; Fernandes et al., 2023).
Host. Lepidoptera: Depressariidae (Gauld, 2000; Yu. et al., 2016).
Examined material. Colombia, Caquetá: 26♀, 1♂. Cartagena del Chairá, Vereda Tigrera Alta, finca Las Palmeras, 01°17’5”N, 74°49’1” W, 235 m[metros], 21.XII.2016- 04.I.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem El Doncello, Vereda La Arenosa, finca El Carmen , 01°40’30”N, 75°16’03” W , 322 m[metros], 18.I.-01.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Valparaíso, Vereda La Florida, finca La Florida, 01°10’18”N, 75°38’40” W, 235 m[metros], 28.X.- 09.XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 23.XI.-07.XII.2016, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA), Florencia, Vereda San Francisco San Pacho, finca El Recreo, 01°42’24”N, 75°36’36” W, 643 m[metros], 01-15.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Solano, Vereda Las Mercedes, finca La Ceiba 01°42’24”N, 75°36’36”, W, 211 m[metros], 29.III.-12.IV.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Cartagena del Chairá, Vereda Tigrera Alta, finca Las Palmeras, 01°42’24”N, 75°36’36” W, 235 m[metros], 01-15.II.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem San José del Fragua, Vereda Bellavista, finca Mi Ranchito, 01°18’23”N, 76°00’32” W, 265 m[metros], 26.X.-09.XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Cartagena del Chairá, Vereda Tigrera Alta, finca Las Palmeras, 01°17’5”N, 74°49’1” W, 235 m[metros], 21.XII.2016-04.I.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem (1♀, LEUA); idem (1♀, LEUA); idem Albania, Vereda Florida 1, finca El Jardín, 01°15’08”N, 75°53’05”W, 283 m[metros], 04-18.IX.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Belén de los Andaquíes, Vereda Aletones, finca La Cabaña, 01°29’31”N, 75°52’20” W, 369 m[metros], 01-15.III.2017, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem 26.X.-09.XI.2016, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA), idem Valparaíso, Vereda La Florida, finca La Florida, 01°10’18”N, 75°38’40” W, 235 m[metros], 26.X.-09. XI.2016, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); Albania, Vereda Florida 1, finca El Jardín, 01°15’08”N, 75°53’05”W, 283 m[metros], 04-18.IX.2017, trampa Malaise en cultivo de caña Sacharum officinarum, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda La Viciosa, CIMAZ Macagual, 01°29’55”N, 75°39’25” W, 249 m[metros], 18.I.-01.II.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); idem 23.XI.-07.XII.2016, (1♀, LEUA); idem Florencia, Vereda paraíso, finca Paraíso, 01°44’51.4”N, 75°37’46.8”W, 663 m[metros], 04-23.XI.2016, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA), idem San José del Fragua, Vereda Bellavista, finca Mi Ranchito, 01°18’23”N, 76°00’32” W, 265 m[metros], 07-21.XII.2016, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem Belén de los Andaquíes, Vereda Aletones, finca La Cabaña, 01°29’31”N, 75°52’20”W, 369 m[metros], 29.III.-12. IV.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA), Florencia, Vereda La Viciosa, CIMAZ Macagual, 01°29’55”N, 75°39’25” W, 249 m[metros], 18.I.-01.II.2017, trampa Malaise en bosque secundario - suelo, Y. Ramos-Pastrana (1♀, LEUA); Albania, Vereda Florida 1, finca El Jardín, 01°15’08”N, 75°53’05”W, 283 m[metros], 04-18.I.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA); idem San José del Fragua, Vereda Bellavista, finca Mi Ranchito, 01°18’23”N, 76°00’32” W, 265 m[metros], 23.XI.-07.XII.2016, trampa Malaise en bosque secundario - dosel, Y. Ramos-Pastrana (1♀, LEUA); idem Florencia, Vereda La Viciosa, CIMAZ Macagual, 01°30’32”N, 75°40’28” W, 253 m[metros], 18.I.-01.II.2017, trampa Malaise en cultivo de caña (Sacharum officinarum), Y. Ramos-Pastrana (1♀, LEUA).
Key to genera of Cremastinae (Hymenoptera: Ichneumonidae) from Caquetá, Colombia [adapted from Gauld (2000)]
1. Tergite II of metasoma with thyridium (Fig. 2A, C, D) ... 2
Tergite II of metasoma without thyridium (Fig. 3B) ... 4
2. The thyridium is located very close to the anterior margin of tergite II, separated from it by a distance equal to its maximum diameter (Fig.2 A). In ventral view, the first segment of metasoma always exhibits widely separated and subparallel tergite margins (Fig. 4F) ... PristomerusCurtis, 1836
Thyridium separated from the anterior margin of tergite II by a distance greater than its greatest diameter, often about 0.5 distance along the tergite (Fig. 2C, D) rarely, separated from the anterior margin by a distance slightly greater than its diameter ... 3
3. The scutellum with strongly raised lateral longitudinal carinae (Fig. 2E), the mandible is slightly curved, with a wide ventral edge (Fig. 3A), and the female has a very short and strongly downward curved ovipositor (Fig. 3C) ... CreagruraTownes, 1971
The scutellum without any trace of lateral longitudinal carinae (Fig. 2F), the mandible is not curved, without obvious ventral border (Fig. 3B), and the female has a long ovipositor, at least as long as the hind tibia, and basally straight or slightly sloping upwards (Fig. 3D) ... XiphosomellaSzépligeti, 1905
4. Tergite II with pendent laterotergite, membranous (Fig. 2B, C, D) ... 5
Tergite II with ventrally folded laterotergite (Fig. 2A) ... 6
5. The fore wing with 1m-cu and Cu1a separated basally by an abscissa (Fig. 3E), the hind femur almost always has a sharp ventral tooth (Fig. 4A), the fore wing with discernible petiolate areolet or distinct 2rs-m, and the upper end of epomia is widened and flared into a triangular rim adhering to the back of the head (Fig. 4C) ... EiphosomaCresson, 1865
The fore wing has 1m-cu and Cu1a basally attached (Fig. 3F), the hind femur lacks a ventral tooth (Fig. 4B), the fore wing with 1m-cu joined to M at its junction with Rs, with no trace of 2rs-m, 3rs-m or areolet, and the upper end of epomia is not widened (Fig. 4D) ... EutanygasterCameron, 1911
6. The first segment of metasoma is distinctly thin, with the margins of tergite I arched to approach or even touch medioventrally, partially hiding the sternites (Fig. 4E) ... TemeluchaFoerster, 1869
The first segment of metasoma is moderately slim, with the margins of tergite I parallel and widely separated, exposed the sternites along their entire length (Fig. 4F) ... TrathalaCameron, 1899
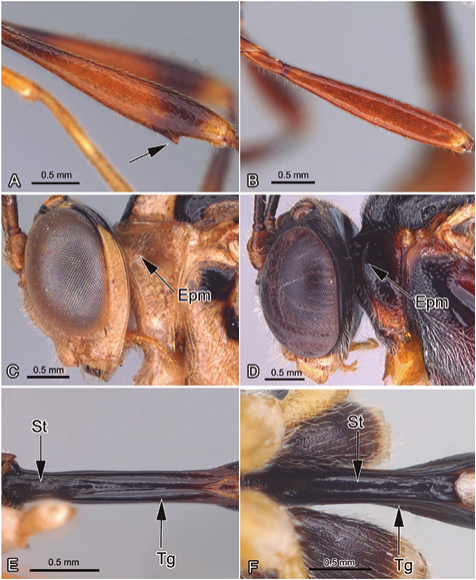
Figure 4 A. Eiphosoma sp. Femur with ventral tooth; B. Eutanygaster sp. Femur without ventral tooth; C. Eiphosoma sp. Pronotum and head, lateral view; D. Eutanygaster sp. Pronotum and head, lateral view; E. Temelucha sp. First metasomal segment, ventral view; F. Trathala sp. First metasomal segment, ventral view. Abbreviatios: Epm: Epomia; St: Sternite; Tg: Tergite.
Source: own elaboration.
Figure 5 Geographical records of Creagrura, Eutanygaster, Pristomerus, Temelucha and, Trathala from Colombia.
Discussion
This study contributes to regional and national entomology by providing the first record of the genera Creagrura, Eutanygaster, Pristomerus, Temelucha, Trathala and Xiphosomella in Colombia.
The record of these genera highlights the significance of conducting faunal inventories and studying the distribution and diversity of Darwin wasps (Narolsky, 2002), particularly in poorly sampled areas like the Colombian Andean-Amazonian region. It highlights the need for comprehensive research to accurately determine the distribution of genera and species throughout the country.
Notably, Eiphosoma and Xiphosomella were the most abundant genera observed, indicating that they can be readily collected in foothills and Amazon plain forests. Despite conducting an extensive six-month sampling effort across various habitats, the other genera, although their rarity or difficulty of collection could not be definitively determined, exhibited very low abundance, including the sugarcane crops, using different sampling methods, with 20 sampling stations distributed throughout the department (Parada-Marín, et al., 2021). Creagrura, Eutanygaster, Pristomerus, and Temelucha genera were exclusively collected in sugarcane crops, but Eiphosoma, Trathala and Xiphosomella were not only collected in sugarcane crops but also from secondary forest in both the canopy and understory.
Conclusions
This study highlights the significance of conducting inventories of understudied groups like Cremastinae, as it contributes valuable scientific findings that enhance our understanding of the country’s biodiversity. These findings serve as a foundation for future research in areas such as taxonomy, systematics, ecology, biogeography and conservation.
The newly recorded genera in this study expand our knowledge of the New World geographical distribution and habitat preferences of Cremastinae in the previously unexplored Andean-Amazonian region of southeastern Colombia.
Furthermore, the dichotomous key to the genera of Cremastinae provided in this study will facilitate their identification by illustrating critical characters and presenting relevant diagnostic information to distinguish them from other genera.