INTRODUCTION
The impacts of the COVID-19 pandemic, whether on the economy, education, and socialization, but mainly the loss of millions of lives, are immeasurable. 1-3 Thus, measures such as facemasks, social distancing, tests for tracking patients, and even lockdowns have been implemented worldwide to contain the spread of the virus.
Furthermore, a race started to develop drugs for treating patients with COVID-19 and, above all, vaccines that can interrupt viral transmission through collective immunity and avoid the occurrence of moderate to severe forms of the disease, reducing morbimortality. 3-7.
Vaccines have absolute value in health promotion and disease prevention. 5,8 However, their development is characterized by low public investment and a 10 to 30-year long process for pre-clinical stages and clinical tests to reach large-scale production after approval and licensing. 2,3,7,9 In contrast, the development of COVID-19 vaccines has been characterized by generous public funding, speed, and an unimaginable number of COVID-19 vaccines candidates. 3,8 A comparison of the time flow, steps, and investment required for traditional vaccine development versus the current flow of COVID-19 vaccines is shown in Figure 1. 2,7,10-12.
Figure 1 shows an accelerated process for developing COVID-19 vaccines, going from up to 20 years to reach vaccination in a commercial model to about nine months for the first person in the world to be vaccinated against COVID-19 in the United Kingdom, while still in the clinical trial stage. 2,7,10,11,13,14 The traditional vaccine development process is characterized by stages with well-defined objectives, as shown in Table 1. 2,10-12.
The accelerated development of COVID-19 vaccines, which has allowed the rapid implementation of immunization plans, can be explained in part by public and non-profit institutions' massive investment in companies with candidate vaccines and the emergency use authorization issued by the World Health Organization (WHO). 3,4 As of November 2021, 20 months after the WHO characterized COVID-19 as a pandemic, the situation of COVID-19 vaccines was as follows: 329 vaccine candidates, of which 111 were in clinical testing, 23 in use, and seven had already received the WHO emergency use authorization and are still undergoing clinical trials. 10,11,15 In addition to the WHO, countries also have the autonomy to issue emergency use authorizations for vaccines, whether or not authorized by the WHO; consequently, the number of vaccines currently in use in the world is greater than the number of vaccines authorized by the WHO. 10,15.
EMERGENCY USE AUTHORIZATION IN PUBLIC HEALTH
An emergency use authorization can be defined as an action by a health regulatory agency, whether national, multinational, or global, to employ unapproved medical products, including vaccines, during public health emergencies. 8,16 However, for an emergency use authorization to be deliberate, there should be no other suitable and safe alternatives, be it diagnosis or treatment, already available and approved by the public health regulator. 8,16 Once these requirements are met, it is still necessary for the company interested in making the health product available for emergency use to submit it to the health regulator. 8,16 Subsequently, the regulatory agency may, according to its internal requirements, determine whether the health product can be approved for emergency use or not. 8,16.
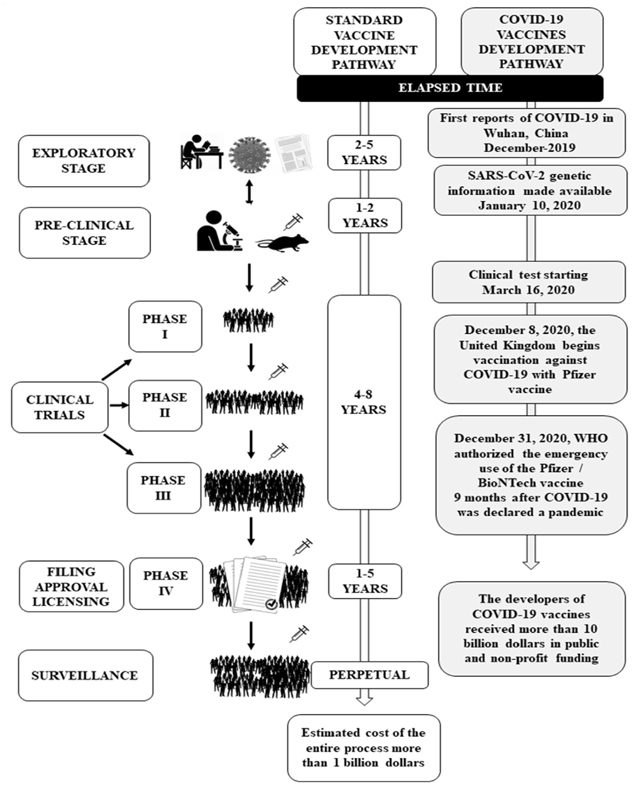
Source: Own elaboration.
Figure 1 Comparison between traditional and COVID-19 vaccine development pathways.
Table 1 Steps and objectives of the traditional vaccine development process
| Vaccine development stage | Objectives | |
|---|---|---|
| Exploratory | To study pathology from the viewpoint of biological structure, the pathogen's genetic sequencing and mechanism, and its course and clinical characteristics, including potential drugs with some therapeutic action and epidemiology. To identify possible antigens. | |
| Pre-clinical | To assess the capacity to induce an immune response (immunogenicity) and safety using cell cultures and animal tests. To assess the appropriate route of administration, adjust the dose, and determine Good Manufacturing Practices to produce batches for phase II tests. To characterize the antigen and assess toxicity. To investigate cellular response and potential immunity mechanisms. | |
| Clinical Trials | Phase I | To assess safety, dosage variations, and immunogenicity, including the extent and type of immune response, in a small group of adults. |
| Phase II | To assess to immunogenicity, safety, dosage variation, efficacy, and variations in the immunization schedule in hundreds of individuals, including risk groups with the pathology. | |
| Phase III | To assess immunogenicity, efficacy, and safety, mainly regarding rare adverse events, in a large group of individuals, preferably a multicenter study. | |
| Filing, Approval, and Licensing | If preliminary tests are successful, the vaccine manufacturer will apply for a license from the regulatory health agency, which inspects the manufacturing facilities. To assess the cost-benefit of the immunizer and its possibility of implementation. If the licensing is approved, the manufacturer undergoes inspections, test reviews periodically, and vaccine batch analyses by health regulatory agencies, including Phase IV tests (Pharmacovigilance Measures) | |
| Phase IV | To monitor safety, efficacy, and other possibilities of use, incorporating pharmacovigilance measures | |
| Surveillance | To assess, notify, monitor, and implement a database regarding adverse events of vaccines. | |
Source: Own elaboration.
Similarly, the entire context mentioned above applies to an emergency authorization of COVID-19 vaccines since the COVID-19 pandemic constitutes a public health emergency, where there is no approved or adequate health product for its prevention and treatment.
Thus, the WHO emergency use authorization for COVID-19 vaccines is a risk-based procedure that assesses vaccine candidates to allow both United Nations agencies and their member countries to purchase vaccines previously approved by the WHO on criteria related to safety, quality, performance, and efficacy. 17,18
Furthermore, COVID-19 vaccine manufacturers are under constant surveillance to commit themselves to standards of excellence in the manufacture and distribution of vaccines. 17,19 Thus, the main objective of the WHO emergency use authorization for COVID-19 vaccines is to accelerate the availability of vaccines for immediate use in immunization plans. 18 Table 2 presents the eligibility criteria, analyzed aspects, and post-authorization guidelines that a vaccine candidate must meet until obtaining its emergency use authorization from the WHO. 17,18.
Table 2 Eligibility criteria, assessed aspects, and post-authorization guidelines in the WHO emergency use authorization for COVID-19 vaccines
| Eligibility criteria for the WHO emergency use authorization of COVID-19 vaccines | Emergency use authorization applies to cases where there is a severe illness, implying an immediate risk of life. The disease must have the potential to cause an outbreak, epidemic, or pandemic, with no licensed products for indication or existing vaccines and drugs able to eradicate the disease or prevent outbreaks The vaccine must be manufactured following current Good Manufacturing Practices. The requesting company must commit to completing product development and require prequalification by the WHO as soon as the vaccine is licensed. |
| Aspects assessed by the WHO | As vaccines are still in development (unlicensed), the WHO will assess quality, safety, and efficacy (or performance) in a risk-benefit analysis using data generated during development to decide whether such vaccines can be used outside clinical trials. For a COVID-19 vaccine candidate to have its emergency use approved, it must prove through consistent data that the benefit to the target population outweighs the risks. |
| Guidelines after the WHO emergency use authorization for COVID-19 vaccines | If a WHO signatory nation approves a vaccine, there will be no duplicate evaluation, but the WHO will evaluate that vaccine for quality, safety, efficacy, and performance criteria. Emergency use authorization only aims to make vaccines immediately available to the population. Thus, as vaccines are still in the clinical testing phase, manufacturers are required to continue the traditional vaccine development process until reaching definitive licensing eventually, if all previous steps are successful. The companies that develop COVID-19 vaccines must submit a risk management plan and appropriate guidelines for the population. The surveillance of COVID-19 vaccines will be permanent. |
Source: Own elaboration.
Therefore, based on the aspects referred to in Tables 1 and 2, the authorization process for emergency use of COVID-19 vaccines can be practically accelerated through cooperation between international health agencies and between these and the WHO, avoiding multiple duplicate analyses, and with the planning and performance of phase II and III tests even before phase I is finished and, preferably, in several countries simultaneously. 2,6,7,10-12,17,18 Thus, the benefit of obtaining vaccines in a short time in a pandemic crisis is undeniable. However, the distribution and immunization of the population with vaccines in the clinical testing stage through emergency use authorization requires special ethical attention and permanent surveillance in every process, whether in the pre-clinical and clinical testing phases or after licensing. Figure 2 highlights the importance of surveillance in the development process and immediate availability of COVID-19 vaccines through emergency use authorization.
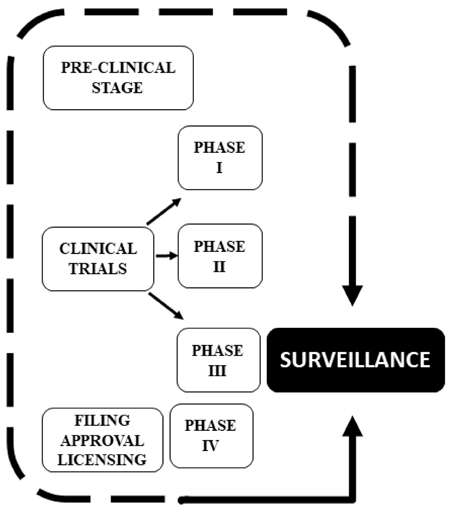
Source: Own elaboration.
Figure 2 Diagram represents the importance of surveillance in the development and immediate availability of COVID-19 vaccines through emergency use authorization.
Note that Figure 2 proposes that surveillance ceases to be characterized as the last stage in the vaccine development process as shown in Figure 1 and becomes one of the fundamental requirements of pre-clinical and clinical tests so that ethics requirements are effectively met.
BIOETHICS APPLIED TO THE EMERGENCY USE OF COVID-19 VACCINES
Upon explaining that the pandemic situation requires efforts for the rapid implantation of immunization programs, a new ethical discussion is established as the emergency use authorization implies using vaccines, as follows:
In the exploratory stage, many questions remain unanswered regarding the pathogenic mechanism of SARS-CoV-2, variations in patients' clinical courses and mainly the appearance of new strains. 6,20.
The selection of an ideal animal model that reproduces the clinical disease to study the pathogenesis of SARS-CoV-2 could require long-term studies. 2 Contrary to the gold standard for a vaccine, defined as infection prevention, studies in primates have shown a reduced viral load and a lower risk of patient's evolution to severe COVID-19 forms. 6 In addition, choosing an animal model that does not allow a direct correlation with humans can constitute an impediment to obtaining approval for animal research from the ethics committee since there would be no glimpse of beneficence in infecting animals to favor science.
Vaccine tests in phases I and II generally involve adults without comorbidities, which, according to current evidence, would not be an ideal representative group for the population at greatest risk of morbimortality for COVID-19. Likewise, questions regarding adverse reactions, durability, and immune response may not be sufficiently answered even in phase III studies if the time factor is reduced. In addition, the wide availability of COVID-19 vaccine candidates for clinical trials implies the concomitance of population immunization cycles and phase I, II, and III tests, impacting research results. 2,6,10-12
Preliminarily, before the bioethical approach, there is a need to identify the two individuals involved in the vaccine development cycle and immunization plan. In the traditional pathway, the individual who receives a vaccine still in clinical tests is called a volunteer and, therefore, voluntarily decides to participate by completing a consent form stating the potential risks and benefits of vaccination, accompanied by researchers. In turn, in the immunization cycle resulting from the emergency use of COVID-19 vaccines, there are one or more vaccines under clinical tests, which have already been included in the immunization plan of a particular country in a global attempt to contain the pandemic, where population and their individuals will be the recipients. Therefore, the vaccine development stage is the same in both cases (the clinical testing phase), but the pandemic context requires ethical reflection. since a COVID-19 vaccine may be non-mandatory.
It is also necessary to define the level of surveillance necessary for the individual, especially regarding potential late adverse effects, which goes beyond the limits of beneficence and non-maleficence. 5 In addition, in some countries, clinical trials of new vaccines, that is, the research modality of a COVID-19 vaccine candidate, can coexist with a fully functioning vaccination plan employing a vaccine with emergency use authorization, which mainly tends to increase bioethical complexity. 6 Therefore, the basic principles of bioethics referring to autonomy, beneficence, non-maleficence, and justice must be approached from the pathway for mass immunization of the population supported by emergency use authorization of vaccines.
Autonomy in the context of the emergency use of COVID-19 vaccines
Utilitarianism, liberalism, and communitarianism are three theoretical aspects related to the ethical context of the traditional use of vaccines and, therefore, can be a matter of discussion when it comes to the emergency use of COVID-19 vaccines. 5 Utilitarianism is a consequentialist approach, which advocates that vaccination policies and actions be intended to achieve the most significant possible impact on general well-being. 5,21 In this context, utilitarianism could justify the mandatory vaccination to health professionals where there would be a tremendous social impact. It would preserve pro fessionals at the forefront of the COVID-19 care and at significant risk of contamination and transmission of SARS-CoV-2, while guaranteeing the service to the population, constituting a more significant impact on the community as a whole. 5,6,21,22 However, Bowen and the WHO suggest that mandatory vaccination for health professionals may oppose medical ethical precepts related to autonomy, such as self-determination, which could affect confidence in vaccination. 6,21
In liberalism, it is the individual who determines the benefit of vaccination for himself, while, in communitarianism, a community benefit from vaccination would be prioritized. 5 In this sense, Afolabi considers liberalism and communitarianism as unethical postulates intended merely to justify social conduct, whether in the sense of vaccination hesitation without justifiable reason or in the sense of making vaccination mandatory when immune response and safety remain incompletely elucidated. 5 Therefore, in a pandemic, with the non-equal distribution of vaccines globally, deciding on autonomy in the emergency use of COVID-19 based strictly on theories can entail serious bias. Each theory has fundamentals that, separately, can result in mandatory measures or not. 5,6,21
A moderate approach to autonomy as proposed by the WHO to balance community and individual well-being may be appropriate in the context of the emergency use of COVID-19 vaccines. 22 In its policy brief dated May 13, 2021, the WHO has highlighted that it does not support any measures for mandatory COVID-19 vaccination, including international travel, and, in particular, does not encourage mandatory systems based on threats or restriction of rights such as working or studying, which may otherwise undermine vaccine confidence.22 Nowadays, the WHO has advised investment in information campaigns to establish confidence in COVID-19 vaccines and policies that make vaccination accessible. 22
Another aspect that can enhance autonomy and remain aligned with the Universal Declaration on Bioethics and Human Rights (UDBHR) published in 2005 23, in the context of the emergency use of COVID-19 vaccines, is to request free prior informed consent for vaccination, whether such vaccines reach the plans of immunization categorized based on the traditional vaccine development pathway in a clinical trial stage or their immune response, safety and efficacy is not fully understood. 6,23 It is noteworthy that Article 6 of the UDBHR clarifies that any preventive medical intervention, where the vaccine is included, necessarily requires a consent form 23. Furthermore, when clarifying the risks and benefits of vaccination mainly in emergency use and allowing the individual's free choice, the consent form contributes to respecting human dignity, human rights, fundamental freedoms, and autonomy, as postulated in Articles 3 (Human dignity and human rights), 4 (Benefit and harm), 5 (Autonomy and individual responsibility), and 6 (Consent) of the UDBHR 23. Thus, good practices in observing Articles 3 to 7 of the UDBHR are available from the Mid Essex Clinical Commissioning Group, National Health Service of the United Kingdom (NHS) through different consent forms in language accessible to the entire population (Table 3). 23-25
Table 3 COVID-19 vaccination consent forms: Categories, target audience, and main characteristics
| CONSENT FORM CATEGORIES | TARGET AUDIENCE | MAIN CHARACTERISTICS |
|---|---|---|
| Adults able to consent themselves | Consent form for adults able to consent | †, ‡ |
| Care homes | Consent form for adults able to consent | †, ‡ |
| Consent form for care home residents able to consent | †, ‡ | |
| Consent form for the attorney of a care home resident unable to consent for themselves | †, ‡,§ | |
| Consent form for a relative of a care home resident unable to consent for themselves | †,‡, ¶ | |
| Health and social workers | Consent form for frontline social workers | †, ‡ |
| Consent form for healthcare workers | †, ‡ | |
| Consent form for social workers | †, ‡ | |
| Housebound patients | Consent form for the attorney of a housebound patient unable to consent for themselves | †,‡, § |
| Consent form for a Relative of a housebound patient unable to consent for themselves | †,‡, ¶ | |
| Easy-read consent form for adults | Easy-read COVID-19 vaccination consent form for adults able to consent | †, ‡ |
† It presents detailed information on pregnancy, planning pregnancy, or breastfeeding. The informative material suggests that pregnant women have Pfizer / BioNTech or Moderna vaccine due to studies in these groups in other countries without severe adverse reactions.
‡ If the option is not to receive vaccination, the respondent is asked for the reason.
§ The signatory is the health and welfare attorney responsible for the individual unable to consent for themselves.
¶ The signatory is the relative of a care home resident unable to consent for themselves.
Source: Own elaboration.
According to Table 3, to adequately comply with the precepts of Articles 3 and 6 of the UDBHR, the NHS has prepared multiple consent forms, even with inclusive language and the possibility of consenting by a health and welfare attorney or a relative of individuals unable to consent for themselves, ensuring compliance with Article 7 (Persons without the capacity to consent) of the UDBHR. 22-24 Furthermore, special attention is given to pregnant, planning pregnancy or breastfeeding. Since vaccines in their initial testing stages do not address these groups, some adverse risks may still be unknown, which ensures compliance with article 8 ( Respect for human vulnerability and personal integrity) of the UDBHR. 4
In turn, the admission of surveillance is an essential requirement for the emergency use of vaccines. The pre-clinical and clinical testing stages can contribute to autonomy; they could provide relevant data regarding potential adverse effects and safety, building confidence in individuals and the community in vaccines, increasing adherence to the immunization plan, and combating vaccine hesitation. In addition, the large availability of vaccine candidates, which have different development platforms, suggests that the identification of an adverse effect on a given vaccine would not imply the impossibility of being vaccinated. It would mean that the individual or a specific group of individuals prone to such adversity could be immunized with another vaccine that has not had such an adverse reaction.
Non-maleficence in the context of the emergency use of COVID-19 vaccines
The COVID-19 pandemic as a global humanitarian crisis involves complex ethical dilemmas such as reaching a balance between not causing harm or non-maleficence and bringing a benefit to others or beneficence. 5,6,21,26,27 As Vashishtha and Kumar and Law and Lo suggested, in the current state of emergency use of COVID-19 vaccines, it is not possible to admit that the principle of non-maleficence is fully satisfied, given that adverse effects may be unknown. A large number of COVID-19 vaccines carries the risk of different adverse effects for each vaccine development platform, while messenger RNA-based vaccine platforms have not previously been approved for use in humans. 6,26 Thus, surveillance can play an important role in preventing serious or late adverse effects from being neglected, keeping the community informed, building trust, and contributing to non-hesitation regarding vaccination.
On the other hand, if it is considered that the rush to buy COVID-19 vaccines that some countries promote may cause an imbalance, as other nations have their access to vaccines curtailed and their population at risk then it will constitute maleficence. 2,4,5 COVID-19 Vaccine Global Access Facility (COVAX), co-led by the Vaccine Alliance (Gavi), the Coalition for Epidemic Preparedness Innovations (CEPI), and the WHO 3,4,28,29, plays a vital role in accelerating the development and production of safe vaccines and providing equal access to the vaccine for all nations. Therefore, by encouraging international cooperation for the development and availability of the COVID-19 vaccine to all nations, COVAX seeks to contemplate UDBHR interrelated principles of equality, non-discrimination, respect for pluralism, solidarity and cooperation, social responsibility and health, transnational practices and international cooperation. 23 At the same time, by supporting research and development of safe and effective COVID-19 , COVAX follows the precepts of Article 4 - Benefit and harm of the UDBHR 23, maximizing the direct and indirect benefits for individuals and minimizing any possible damage.
Therefore, making vaccines equally accessible to all peoples and ensuring access to information and surveillance about risks can contribute to non-maleficence in the emergency use of COVID-19 vaccines.
Beneficence in the context of the emergency use of COVID-19 vaccines
Beneficence, in the context of bioethics, implies caring for others. Be it for the guarantee of rights, prevention of risks and damages, or accessibility of others to the vaccine, the meaning will always be a vision focused on protecting another individual, community, or nation. 5,6,21,27 Like non-maleficence, the equal distribution of vaccines would be fundamental for beneficence, either by local herd immunity or by preventing the spread of SARS-CoV-2 globally. 3 However, the initial proposal of COVAX, which would be to immunize risk groups preliminarily and equally, which would correspond to 20 % of the population and, subsequently, other groups in all nations cannot be reached. Some countries with high economic power have started direct negotiations with manufacturers to increase immunization internally in their countries. 3 Since this race will inevitably lead to high vaccination rates in some countries, even if late, it will favor COVAX by putting less pressure on manufacturers for vaccine delivery and other COVID-19 vaccines entering the market and obtaining approval.
Otherwise, one has to consider that the benefit of using a COVID-19 vaccine in terms of the gold standard would mean its ability to prevent COVID-19 infection. 6 However, for COVID-19 vaccines, a benefit for emergency use has been admitted based on the criteria of viral load reduction and protection of the individual against severe COVID-19 forms. 6,26 In fact, no COVID-19 vaccine will provide 100 % effectiveness, with current studies ranging from just over 50 % to 95 %, implying a need for the already vaccinated population to be informed about maintenance, testing measures, social distance, hand hygiene, facemasks, and seeking medical care in case of suspected COVID-19, as there are reports of reinfection. 2,4,6,26
Again, it is noteworthy that surveillance plays a vital role in beneficence since it can identify risks of adverse reaction over time, intervene early in cases that require limiting damage, and keep the population informed to build confidence in the vaccine.
Justice in the context of the emergency use of COVID-19 vaccines
Justice in the bioethical environment implies offering equal opportunities to all, seeking to avoid as much as possible any burden on the parties involved, whether from a personal or social point of view. 5,6,27 Thus, in the emergency use of COVID-19 vaccines, personal justice takes place when the individual is vaccinated, avoiding a burden on society, either by the spread of the pandemic or by the need to allocate greater resources for treatment in case it evolves to a serious form. 5,27 Otherwise, social justice in the scenario of COVID-19 immunization can be achieved through awareness campaigns about the importance of vaccination or by making immunization mandatory to contain the spread of the SARS-CoV-2. 5,27 However, adopting balanced measures between the personal and the social can encourage immunization and avoid strife since, even under mandatory conditions, vaccination hesitation can persist. 5,27 In this context, hesitation related to emergency use of COVID-19 vaccines has been related to how rapid vaccines have gone through the development process for use in the population, especially messenger RNA platform vaccines without precedent for use in humans, and the emergence of conspiracy theories on social media fueled by the politicization of vaccines.
Therefore, surveillance can contribute to justice by seeking a better allocation of resources for promoting population's confidence in and adherence to immunization plans against COVID-19. Surveillance ensures government control over the effectiveness and safety of vaccines. In addition, measures to collect information about the reason for hesitation, as practiced in the NHS consent form, can assist in directing broad awareness campaigns that improve population's confidence in and adherence to the emergency use of COVID-19 vaccines.
CONCLUSIONS
This paper presents an approach to the bioethical precepts of autonomy, non-maleficence, beneficence, and justice in the context of the emergency use of COVID-19 vaccines. Furthermore, surveillance is highlighted as a fundamental requirement from clinical and pre-clinical testing to post-licensing. Surveillance contributes to the observance of bioethical precepts, especially the monitoring and adoption of early measures in case of adverse effects during the emergency use of vaccines, playing a role in promoting the population's trust in immunization plans. However, the new paradigm in vaccine development determined by COVID-19, characterized by a high speed, many vaccine candidates, availability of new platforms, and the possibility of emergency use to respond to viral spread worldwide, requires expanding the debates about the bioethical challenges arising from the implementation of immunization plans against COVID-19.















