Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Biotecnología
Print version ISSN 0123-3475
Rev. colomb. biotecnol vol.14 no.1 Bogotá Jan./June 2012
ARTÍCULO DE INVESTIGACIÓN
Assays for the in vitro establishment of Swietenia macrophylla and Cedrela odorata
Ensayos para el establecimiento in vitro de Swietenia macrophylla y Cedrela odorata
Julián Pérez Flores 1, María Elena Aguilar Vega 2, Robert Roca Tripepi 3.
1 Colegio de Postgraduados - Campus Tabasco, Km. 3 Carretera Cárdenas - Huimanguillo, H. Cárdenas Tabasco México, CP 86500 julianflores@colpos.mx Author for correspondence.
2 Tropical Agricultural Research and Higher Education Center (CATIE), Turrialba 7170, Costa Rica.
3 University of Idaho, Department of Plant, Soil, and Entomological Sciences, Moscow, Idaho, 83844, USA. Running head: Establishment of Mahogany and Spanish cedar nodal explants
Recibido: febrero 09 de 2011 Aprobado: junio 06 de 2012
Abstract
Recalcitrance and contamination in Mahogany (Swietenia macrophylla King) and Spanish cedar (Cedrela odorata L.) stem tissues are the main causes of its ineffective in vitro propagation. The objectives of this research were: a) to evaluate sodium hypochlorite (NaOCl) and plant preservative mixture (PPM®) as surface disinfectants and/or added to the culture medium for the in vitro establishment of nodal explants taken from 10-year-old Mahogany and Spanish cedar plants, and b) to evaluate the in vitro response of such explants treated with N6-benzylaminopurine (BAP) (0, 2.2, 4.4, 8.8, 17.7 μM), silver nitrate (AgNO3) (0, 3 mg l-1), activated charcoal (0, 1 g l-1) and vented caps. All the experiments were arranged in a completely randomized design. The NaOCl at 15%, for 20 min, as a surface sterilization or PPM® at 2 ml l-1 into the culture medium, were the best treatments to reduce contamination for both species. For Mahogany explants, BAP at 17.7 μM resulted in higher percentages of bud breaks than Spanish cedar (64% and 25%, respectively). Leaves on elongated shoots dropped off by 20 days after starting the explants in culture and neither the activated charcoal nor the AgNO3 alone or combined prevented leaf abscission. The AgNO3 decreased contamination, but also increased leaf abscission. Bud breaks was two-fold higher for nodal explants established in vessels with vented caps than with normal caps. Mahogany nodal explants were easier to surface sterilize and more buds broke from BAP treated explants than Spanish cedar treated explants in the in vitro establishment.
Key words: Spanish cedar, Mahogany, Mature plants, Surface sterilization, in vitro response.
Resumen
La contaminación y la recalcitrancia de tejidos de tallo de Caoba (Swietenia macrophylla King) y Cedro español (Cedrela odorata L.) son las causas principales de su inefectiva micro-propagación. Los objetivos de la investigación fueron: a) evaluar el hipoclorito de sodio (NaClO) y una mezcal preservadora de plantas (PPM®) como desinfectantes superficiales y/o agregados al medio de cultivo para el establecimiento in vitro de explantes nodales de Caoba y Cedro español de 10 años de edad; b) evaluar la respuesta in vitro de tales explantes tratados con N6-benzylaminopurine (BAP) (0, 2.2, 4.4, 8.8, 17.7 μM), nitrato de plata (AgNO3) (0, 3 mg l-1), carbón activado (0, 1 g l-1) y tapas porosas. Los experimentos fueron establecidos bajo un diseño completamente al azar. La contaminación se redujo en ambas especies con NaOCl al 15% durante 20 min como desinfección superficial o con PPM® (2 ml l-1) agregado al medio de cultivo. El mayor porcentaje de brotación de explantes se obtuvo con BAP a 17.7 μM en caoba (64%) comparado con cedro (25%). Los brotes se defoliaron a los 20 días de cultivo y ni el carbón activado ni el AgNO3, solos o combinados evitaron la defoliación. El AgNO3 disminuyó la contaminación, pero incrementó la defoliación. La brotación fue dos veces mayor en los explantes nodales establecidos en recipientes con tapas porosas que cuando se utilizaron tapas normales. Los explantes nodales de Caoba respondieron mejor a la desinfección superficial y a los tratamientos de BAP comparados con los de Cedro español en el establecimiento in vitro.
Palabras clave: Cedro español, Caoba, Plantas adultas, Desinfección superficial, Respuesta in vitro.
Introduction
Mahogany Swietenia macrophylla King and Spanish cedar Cedrela odorata L. (Meliaceae) are included in the CITES list as endangered species. Many attempts have been done to micropropagate mature individuals of both species without success. Such absence of response is known as recalcitrance which is characterized by lack of ability to break bud, absence or negligible growth of sprouted buds, leaf abscission on new shoots and presence of only one elongated petiole (Lee and Rao, 1988). In addition, contamination is a problem that must be overcome in order to micropropagate older forest trees successfully (Maruyama et al. 1989). Specifically, for Mahogany and Spanish cedar the difficulty in obtaining microbe-free cultures starting with explants from mature trees has been related to surface and internal contamination in donor plants (Maruyama 2006).
Contamination appeared to be absent from initial culture, but contamination was present after the fifth or later subcultures of mahogany shoots from pruned 3-year-old trees (Maruyama 2006). During micropropagation of Toona ciliata (Meliaceae) nodal explants from 2-year-old plants, between 17 and 35% of the cultures were contaminated during establishment, even though explants were surface sterilized by soaking in 70% ethanol for 1 min followed by immersion in 1.8% w/v sodium hypochlorite (NaOCl) for 30 min (Mroginski et al. 2003). For Mahogany nodal explants from 2-year-old plants, losses due to contamination during in vitro establishment were 44% even after double surface sterilization in calcium hypochlorite (Ca(OCl)2) at 10% w/v for 20 min and then 8% w/v for 10 min (Flores 2001). Contamination of explants can also be prevented by adding NaOCl to the culture medium (Teixeira et al. 2006). These researchers report that the addition of NaOCl (0.0003% active chlorine) sterilized the culture media, doubled biomass and the number of new shoots on Ananas comosus explants.
Niedz (1998) suggests Plant Preservative Mixture (PPMTM, Plant Cell Technology, Washington DC, USA) be used to reduce endogenous and surface contamination of explants. This product is a heat stable biocide, which kills bacteria and fungi cells, prevents germination of their spores, and potentially eliminates endogenous contaminants without impairing the response of explants in tissue culture (Guri and Patel 1998). These authors state that the active ingredients (isothiazolones-methylchloroisothiazolinone and methylisothiazolinone) in PPMTM interfere the citric acid cycle and the electron transport chain and also inhibit transport of monosacharides and amino acids in fungal and bacterial cells, improving the response of explants.
The response of explants in vitro also could be improved by adding plant growth regulators (PGR) and other substances to the culture medium and modifying the atmosphere inside the vessels. N6-benzylaminopurine (BAP) is the typical PGR used to induce bud break (Krikorian 1994), whereas activated charcoal and silver nitrate (AgNO3) have been used to improve bud break of explants by modifying both the culture medium and the atmosphere in culture vessels (Kitaya et al. 2005). Silver nitrate is a regulator of ethylene biosynthesis, one of the main gases affecting explant response in culture vessels (Fuentes et al. 2000), and has bactericidal activity (Kuvshinov et al. 1999). Activated charcoal is often added to the culture medium to absorb inhibitory substances released by the explant into the medium and vessel headspace and has the additional effect for enhancing air movement in the culture vessels (Kitaya et al. 2005).
The atmosphere in vessels also can be modified by air exchange as is practiced for photoautotrophic micropropagation (Zobayed et al. 2004). Air exchange inside of the vessels depends on the type of sealant and closures used. For genetic transformation of Brassica rappa, cultures sealed with Micropore 3M paper tape increased regeneration from 0 to 5% up to 60 to 80% compared to parafilm mainly by allowing air interchange (Kuvshinov et al. 1999). Recalcitrance and contamination of Mahogany and Spanish cedar stem tissues are the main causes of ineffective micropropagation of both species in vitro by nodal explants excised from mature donor plants. Therefore, the objectives of this research were: a) to evaluate NaOCl and PPMTM as surface disinfectants and/or added to the culture medium for the in vitro establishment of nodal explants taken from 10-year-old Mahogany and Spanish cedar plants, and b) to evaluate the in vitro response of such explants treated with BAP, AgNO3, activated charcoal, vented caps and Micropore paper.
Materials and methods
Plant material and explant preparation
Single nodal explants were taken from stems of 10- year-old Mahogany and Spanish cedar plants, which were maintained in a greenhouse. The shoots were cut early in the morning (ca. 6 a.m.) to obtain turgid tissues. Inside the greenhouse, leaves were severed, and shoots were put in a plastic bag and taken to the biotechnology laboratory at the Tropical Agricultural Research and Higher Education Center (CATIE, Costa Rica). There, plant material was washed with soap 0.03% (FADIS-Quimisol, Costa Rica), and placed under running water for 15 min, before cutting single nodal explants of about 2 cm in length. Nodal explants were then pre-disinfected as follows unless stated otherwise: 1 hour in Benomyl (Piscis, Costa Rica; 2 g l-1) plus Manzate (Pfizer, México; 3 g l- 1) and Agrimycin (Pfizer, México; 1 g l-1); 20 min in Ca(OCl)2 at 10% w/v and 15 min in Ca(OCl)2 at 8% w/v. Explants were treated with Ca(OCl)2 inside the laminar flow hood. Explants were rinsed three times for 30 seconds each with sterile double-distilled water after surface sterilization. Fungicides, bactericides and Ca(OCl)2 treatments were supplemented with Tween 20 (Sigma, St. Louis, MO, USA) one drop for 100 ml of solution.
1. Establishment of aseptic cultures
1.1. Use of NaOCl for surface sterilization and added to the culture media
a) Pre-disinfection in Ca(OCl)2 was changed by using NaOCl (commercial bleach at 3.5% active ingredient) at 15, 30, or 45% (v/v) for 10 or 20 min and then used in combination with NaOCl at 0, 2 or 4% v/v added to the medium. Each one of the 18 treatments consisted of three replications, each one with 10 Spanish cedar nodal explants.
b) For Mahogany nodal explants, NaOCl at 15% (v/v) for pre-disinfection procedure for 10 or 20 min was combined with NaOCl at 2 or 4% (v/v) added to the medium. Each treatment consisted of five replications, each one with 10 explants.
c) From the two latter experiments, the best combination reducing explant contamination and increasing bud break was NaOCl at 2% (v/v) added to the medium and NaOCl 15% (v/v) for 10 min during surface sterilization. Therefore, another experiment consisting of three Mahogany assays of nodal explants 15 days after each other was completed in order to confirm the effect of such treatment. Each assay consisted of four replications. The number of explants introduced depended on the availability of plant material. Therefore, the first assay had 25 nodal explants per replication, the second had 70, and the third one had 45 nodal explants per replication.
In the three experiments, the medium used was Schenk and Hildebrant (1972, SH medium) supplemented with BAP 1 mg l-1. Containers and caps were autoclaved at 1.46 kg cm-2 for 20 min, and the culture medium boiled for 5 to 10 min after heating for 10 min per liter in a microwave before dispensing into the containers. This procedure of culture media sterilization was used for all experiments since media containing NaOCl failed to gell when autoclaved. To be sure that media were free of microbial contamination non-inoculated media were allowed to sit for 10 d in sterile vessels. For the experiments with PPMTM, media and containers were autoclaved at 1.46 kg cm-2 for 20 min.
1.2. Use of PPMTM added to the culture media and combined to the pre-disinfection treatment. a) In order to test the effect of PPMTM added to the culture medium, media were supplemented with 0, 1, 2, 3, or 4 ml l-1 of this biocide and were placed in a chamber. Each dose (treatment) contained five replications, with 10 explants in each one. b) In a parallel experiment, the pre-disinfection in Ca(OCl)2 was changed by rinsing nodal explants in PPMTM at 0 or 2 ml l-1; later, explants were rinsed with sterile double distilled water and inoculated directly into the culture medium containing PPMTM at 0, 0.5, 1.0, 1.5, or 2 ml l-1. Each treatment had five replications, with five explants in each one.
1.3. Use of PPMTM instead of the pre-disinfection treatment. To reduce internal contamination in Spanish cedar nodal explants, PPMTM at 5% v/v was added to Murashige and Skoog (MS, Murashige and Skoog, 1962) salts instead of the pre-disinfection treatment (Benomyl + Agrimycin, and then Ca(OCl)2). Nodal explants were treated for 6, 12, 18, or 24 h, with the pre-disinfection treatment as control. Gentle shaking was applied continuosly during the PPMTM treatment in a shaker (GyromaxTM 721, HOTECH Instruments Corp., Taipei, Taiwan). Each one of the four treatments and the control included 10 replications, each one with 10 explants per replication. The culture medium was also supplemented with indolebutyric acid (IBA) 0.5 mg l-1, isopentenyladenine (2-iP) 1 mg l-1 and PPMTM 2 ml l-1. Control explants were rinsed three times in sterile double-distilled water after surface sterilization; explants treated with PPMTM at 5% (v/v) were not rinsed.
2. Response of Spanish cedar and Mahogany to in vitro culture
2.1. Effect of BAP on bud break
Spanish cedar and Mahogany nodal explants were surface sterilized by rinsing in fungicides as described above and then treated for 10 min in NaOCl 15% (v/v). One experiment was completed to evaluate the effect of BAP at 0, 2.2, 4.4, 8.8, or 17.7 μM on bud break by both species. Each one of these five treatments had four replications for Spanish cedar and five for Mahogany, each one with 10 explants. The MS culture medium was supplemented with NaOCl 2% (v/v).
2.2. Improvement of the culture atmosphere
a) Use of silver nitrate and activated charcoal. The objective of the experiment was to prevent leaf abscission by shoots from Spanish cedar nodal explants, by adding activated charcoal or AgNO3 to the culture medium. The explants used had been introduced 20 days before on half strength MS medium, supplemented with sucrose 1.5% w/v, 2-iP 3.6 μM, NaOCl 2% (v/v), and Agrimycin 1 g l-1. Activated charcoal at 1 g l-1 and AgNO3 at 3 mg l-1, were tested alone or combined together (three treatments) along with a control, which lacked these substances. Each one of these four treatments included five replications with 10 explants per replication.
b) Use of vented caps. The objective of the experiment was to compare the effects of normal versus vented caps (Magenta Corp., Sigma Chemical) on leaf abscission of Mahogany shoots during in vitro establishment. Three replications were used for each treatment (either a normal or a vented cap on a glass jar) with each replicate containing 50 explants.
c) Use of Micropore tape as a vessel sealant. The objective of the experiment was to compare the effect of Micropore tape (3M Corporation, St. Paul, MN) as vessel sealant during in vitro establishment of Spanish cedar nodal explants compared to using plastic wrap (Glad, Costa Rica) which is a common sealant (control) used around the vessel lid. Six replications were used for both treatments, each one with 12 nodal explants.
Half-strength MS culture medium was used as described by Flores (2001) in the experiments for improving the culture vessel atmosphere of explants in vitro. Medium was supplemented with 1.8 μM 2-iP, 11.4 μM indoleacetic acid (IAA), sucrose 1.5% w/v, activated charcoal 1 g l-1, streptomycin 300 mg l-1, and NaOCl 2% (v/v). The variables evaluated for aseptic establishment of cultures were the percentages of contamination by fungi and bacteria as well as bud break (shoots with axillary buds or at least one expanded bud at least 3 mm long). The response of explants to in vitro culture was recorded as the percentage of leaf abscission (means of fallen leaflets divided per mean total leaves multiplied by 100).
Culture conditions. All culture media were supplemented with sucrose 3% (w/v) unless stated otherwise, and gelled with agar 7 g l-1; the pH was adjusted to 6.0 or 5.8 depending on the addition or absence of NaOCl in the medium. Cultures were incubated at 25 0C under a 12 h photoperiod provided by Phillips cool-white fluorescent light tubes.
Experimental design. All experiments were arranged in a completely randomized design. The experimental unit was an individual nodal explant in a 50 ml vial with 10 ml of culture medium for establishing of aseptic cultures or in 200 ml glass jar with 30 ml of culture medium for the response of nodal explants to in vitro culture.
Statistical analysis. Data were examined for normal distribution and homogeneity of variances required for analysis of variance (ANOVA). Contamination and bud break data were transformed by Y = arcsin(sqrt(Y)), and shoot length was transformed by Y = sqrt (Y + 0.5) before statistical analysis. ANOVA was completed (Proc GLM; SAS, 2001) and, if the F test was significant, means were compared using the Duncan's multiple range test or the least square mean test at the 5% level.
Results and discussion
Although stock plants were maintained in a greenhouse and fungicides and bactericides were applied routinely every 15 d, in a preliminary experiment for in vitro establishment the incidence of contamination was 90 % for Spanish cedar and 84% for Mahogany nodal explants, whereas bud break was lower than 17% for explants from both species. Therefore, the following experiments were completed in an attempt to reduce culture contamination and increase explant bud break.
1. Establishment of aseptic cultures
1.1. Use of NaOCl during surface sterilization and added to the culture media
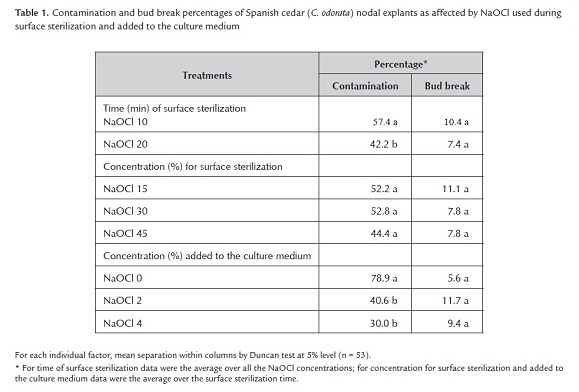
Contamination of Spanish cedar nodal explants differed among levels of NaOCl used during surface sterilization (F17, 36 = 10.03, P < 0.0001), between the two times of sterilization (F1, 36 = 17.33, P = 0.0002), and among levels of NaOCl added to the culture medium (F2, 36 = 66.32, P < 0.0001) (table 1). However, a three-way interaction between the factors was absent (P = 0.1).
A two-way interaction between treatments time for surface sterilization (10 and 20 min) and NaOCl added to the medium (0, 2, 4%) was presented for bud break of explants (F2, 36 = 3.45, P = 0.04). The 20 min of exposure for surface sterilization and NaOCl at 4% (v/v) added to the medium reduced explant contamination, but bud break was similar among NaOCl concentrations (table 1). During the surface sterilization treatments, deep penetration of sterilizing agents into the explant tissue could have killed cells and affected tissues, which resulted in delayed and reduced growth responses by explants (Thakur and Sood 2006), such as bud break.
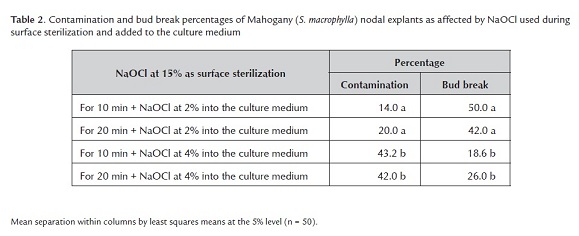
Contamination of Mahogany nodal explants differed among the combination of NaOCl treatments (F3, 16 = 4.15, P = 0.02) (table 2). The NaOCl concentration in the medium affected explant contamination (F1, = 12.09, P = 0.003), and an interaction between the factors was absent (F1 = 0.25, P = 0.6). The surface sterilization time either 10 or 20 min similarly affected both contamination (F1 = 0.25, P = 0.6) and bud break (F1 = 0.25, P = 0.6), and the lower time (10 min) combined with 2% NaOCl added to the medium was enough to reduce contamination to 14%. Combined NaOCl treatments also affected bud break differently (F1, 36 = 7.67, P = 0.0004) (table 2). The NaOCl concentrations in the medium affected bud break (F1 = 28.06, P = 0.0001), and an interaction between NaOCl treatment time during surface sterilization and concentration NaOCl in the medium was absent (F1 = 4.10, P = 0.06).
The lower dose of NaOCl added to the medium favored the higher percentage of bud break. Therefore, the best combination for explant treatment was NaOCl at 2 ml l-1 added to the medium and 15% NaOCl used for 10 min during surface sterilization. Compared to 3% for 10 and 30 min or 2% for 10, 20 and 30 min and in terms of survival, contamination and necrotic explants, Collado et al. (2004) defined NaOCl at 3% for 20 min as the best sterilization treatment for nodal explants from mature S. macrophylla plants.
The least squares means indicated statistical differences among treatments for both contamination and bud break (table 2). The low NaOCl concentration in the medium reduced contamination and increased bud break significantly more than the high concentration in the medium. Such results agreed with those of Teixeira et al. (2006) who reported active chlorine concentration as low as 0.0003% used to sterilize the culture medium improved the response of Ananas comusus (pineapple) by doubling biomass and number of new shoots.
In the follow up study, the best combination of NaOCl treatments was used in three trials for starting Mahogany nodal explants in culture. From these trials, the overall contamination rate of nodal explants was low (19.8% ± 1.8%), and bud break was 53% ± 3.5%. Although bud break was similar among the three trials (F2, 9 = 3.86, P = 0.06), the percentages of explant contamination were 23%, 8.2% and 28.2%, respectively. The contamination differences among the trials were most clearly due to environmental conditions. High contamination rates in the first and third trials were probably due to taking nodal explants during periods of heavy rainfall, and the experiment was conducted in December when temperature was lower than in summer, although rainfall amounts were higher (Salas 2000). For the micropropagation of Eucalyptus tereticornis at various times throughout the year, incidence of endogenous fungal or bacterial contaminants was most severe during the hot and humid months of late summer (Sharma and Ramamurty 2000).
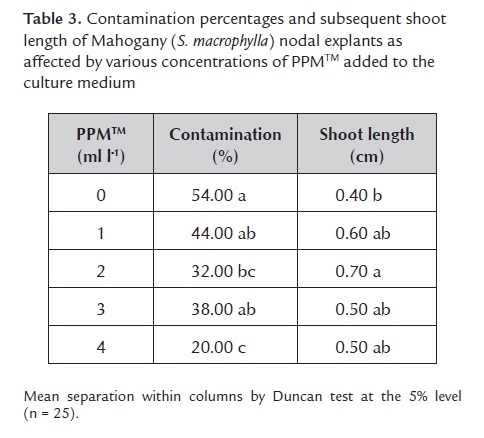
1.2. Use of PPMTM added to the culture medium and then combined with PPMTM treatment during surface sterilization. Various concentrations of PPMTM added to the medium failed to affect contamination (F4, 20 = 0.27, P = 0.9) and bud break percentage (F4, 20 = 1.49, P = 0.2) for Spanish cedar; the mean (± SE) for contamination and bud break were 49.6% ± 3.6% and 71.6% ± 2.5%, respectively, and contamination of nodal explants was mainly due to bacteria. For Mahogany, PPMTM in the culture medium significantly reduced contamination and also significantly favored shoot elongation (table 3), but its effect on bud break was similar (F4, 20 = 0.55, P = 0.7). The mean (± SE) bud break was 81.2% ± 2.4%. Contamination of nodal explants was mainly due to fungi. The difference of contaminants between Mahogany (32.4% fungi, 5.2% bacteria) and Spanish cedar (8.8% fungi, 40.8% bacteria), agreed with results from Leifert and Waites (1992) who showed different plant species grown in vitro create distinct environments inside the culture vessels, which in turn allow or prevent the growth of different contaminants.
Differences of contaminants between both species also could be attributed to different efficacy of PPMTM treatments. The PPMTM added to the medium worked better for Mahogany than for Spanish cedar reducing contamination and increasing bud break. Compared to the preliminary experiment, contamination was reduced by 46.4%, whereas bud break was increased by 68.2% for Mahogany. Besides reducing contamination (F4, 20 = 4.07, P = 0.01) and increasing bud break (F4, 20 = 2.84, P = 0.05), PPMTM at 2 ml l-1 also favored the elongation of sprouted buds on Mahogany (table 3).
For Spanish cedar explants, PPMTM doses had similar effects as the control for contamination and bud break percentages. The mean contamination varied from 42% for PPMTM at 4 ml l-1 to 54% for PPMTM at 3 ml l-1. The bud break mean percentage varied from 64% on PPMTM at 3 ml l-1 to 84% for PPMTM at 1 ml l-1. For the control, contamination and bud break were 50% and 72%, respectively.
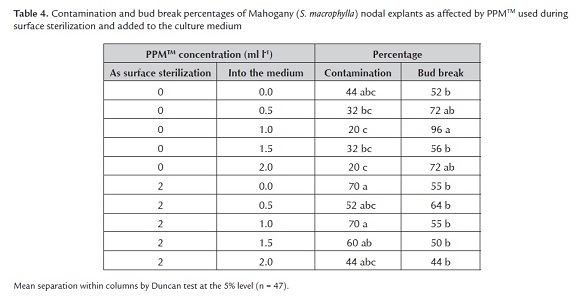
When PPMTM was used during surface sterilization and added to the culture medium, treatment effects on Spanish cedar nodal explants were similar to each other for contamination (F9, 40 = 1.05, P = 0.42) and bud break (F9, 40 = 0.65, P = 0.74). Although the bud break (67.2% ± 3.3%) was similar to that of the previous experiment, the contamination was higher (79.2 ± 2.6%, with 71.5% explants contaminated by bacteria, and 7.7% by fungi). This result could be explained by incomplete pre-surface sterilization treatment for half of introduced explants because PPMTM at 0 or 2 ml l-1 was applied instead of Ca(OCl)2 in the surface sterilization procedure. Jiménez et al. (2006) reported that the use of pre-surface sterilization with NaOCl combined with PPMTM in the culture medium was an efficient way to establish in vitro explants of the neotropical giant bamboo (Guadua angustifolia). Another reason would be that three out of four concentrations of PPMTM added to the medium were lower than the level suggested by the manufacturer (2 ml l-1), which also was the best in the previous experiment to reduce contamination, to increase bud break, and enhance shoot elongation for Mahogany.
For Mahogany, an interaction between surface sterilization with PPMTM and PPMTM in the medium affected explant contamination (F9, 37 = 2.90, P = 0.01) and bud break (F9, 37 = 2.28, P = 0.03) (table 4), but shoot elongation appeared unaffected (F9, 37 = 1.01, P = 0.4). The mean (± SE) shoot elongation was 0.5 cm ± 0.2 cm. If used alone, PPMTM at 1 or 2 ml l-1 added to the medium, controlled contamination better and increased bud break compared to all the other treatments.
The combination of PPMTM used during surface sterilization and added to the culture medium increased contamination and reduced bud break, compared to PPMTM added to the culture medium (the above experiment). In this experiment, PPMTM again reduced contamination more for Mahogany than for Spanish cedar, maybe due to the different microbial contaminants in each species. Mahogany was contaminated by fungi and Spanish cedar by bacteria. Such specificity of contaminants could be attributed to plant species-specific bacteria being introduced into culture due to endogenous contamination within the explants (Fisse et al. 1988).
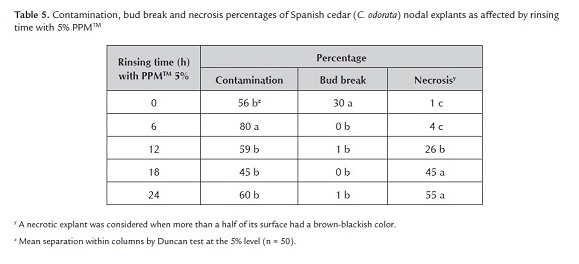
1.3. Use of PPMTM during surface sterilization. Rinsing time with 5% PPMTM during explant surface sterilization affected explant contamination (F4, 45 = 6.28, P = 0.0004), bud break (F4, 45 = 33.18, P < 0.0001), and damage by necrosis (F4, 45 = 45.55, P = 0.0001) depending on the rinsing time (table 5). Compared to the control, PPMTM at 5% during surface sterilization failed to reduce contamination or improve bud break but was toxic to subsequent shoot growth of Spanish cedar nodal explants. Toxicity increased as the PPMTM treatment time increased. Such toxicity could be due to PPMTM by being an acid and inducing necrosis (Guri and Patel 1998). So, PPMTM damaged the nodal explants preventing bud break and increased explant susceptibility to contaminants. Nevertheless, methylisothiazolinone and methylchloroisothiazolinone, the biocides used in PPMTM, are reported to be nonphytotoxic at concentrations of 4 to 5% for 4 to 12 h for the prophylactic control of endogenous contaminants in plant tissue cultures for woody plants (Guri 2004).
Both Mahogany and Spanish cedar responded differently to PPMTM treatments used as rinses for surface sterilization and added to culture media. PPMTM contains two isothiazolones, which are a broad-spectrum industrial biocides (Niedz 1998); however, the level of contamination recorded for both species was similar to that reported by Flores (2001) for nodal explants from 5-year-old Mahogany plants when using Ca(OCl)2 and NaOCl during surface sterilization. This author recorded 44% contamination of explants receiving double surface sterilization treatments with Ca(OCl)2 (10% for 20 min then 8% for 10 min) and 45% contamination with NaOCl at 50% for 15 min. However, donor plants used in this research were 5 years older than those used by Flores (2001), and donor plant age is related to the incidence of endogenous contamination (Maruyama et al. 1989).
The results indicated that PPMTM can be added at 2 ml l-1 to the culture medium during in vitro establishment of Mahogany to control contamination without impairing bud break or shoot elongation. For Spanish cedar, however, more experiments with this product are necessary to reduce contamination. Niedz (1998) indicated that PPMTM can be routinely added to tissue culture medium to control air- and waterborne bacterial and fungal contaminants effectively.
2. Response of Spanish cedar and Mahogany to in vitro culture
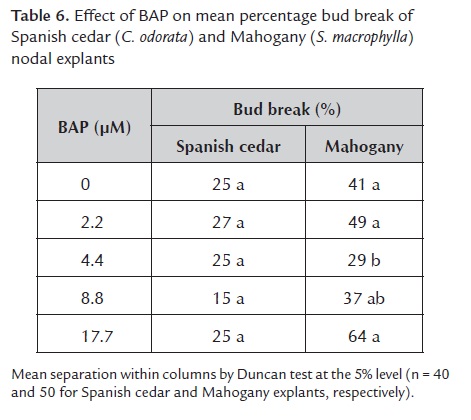
2.1. Effect of BAP on bud break. Various concentrations of BAP failed to affect bud break of Spanish cedar (F4, 15 = 0.91, P = 0.5) or Mahogany (F5, 23 = 1.83, P = 0.1) explants similarly. Nevertheless, both species responded differently to BAP treatments (F5, 39 = 4.94, P = 0.001), and in general bud break was almost twofold higher for Mahogany explants compared to those of Spanish cedar (44.7% ± 3.7% and 23.5% ± 2.1%, respectively). However, different species usually have different responses in vitro (Kane 2000), so the highest BAP concentration resulted in higher percentages of bud break for Mahogany than for Spanish cedar (table 6). This result could be explained because although both species were the same age, Mahogany tissues were woodier than Spanish cedar.
Rodríguez et al. (2003) found a better response for in vitro bud break of a Swietenia hybrid compared to Spanish cedar after BAP treatment. These authors stated that stem explants from both species responded to increasing concentrations of BAP by sprouting more buds; however, Collado et al. (2004) stated that S. macrophylla nodal explants sprouted better at low concentrations (1 or 2.5 μM) of BAP which agreed with this study since there was not statistical difference among treatments (table 6). For Spanish cedar and Mahogany, Pérez et al. (2006) and Flores (2001) respectively, reported that nodal explants responded to 2.2 μM or 6.5 μM better than to 20 μM BAP. The last authors used nodal explants from in vitro seedlings or 2-year-old plants, but here nodal explants from 10-year-old plants were used.
For Mahogany, BAP at 17.7 μM seemed to favor the bud break, which differed with the results of Collado et al. (2004). These authors attained 63.9% bud break on nodal explants from Mahogany by using BAP at 1 μM. However, they did not state the age of their mature plants. Mroginski et al. (2003) also found that BAP at 22.2 μM impair bud break in nodal explants from 2-year-old Toona ciliata (Meliaceae) plants but improved it for explants from 10-year-old T. ciliata plants.
2.2. Improvement of the culture atmosphere. In all the previous experiments, some shoots grew ca. 1 to 1.5 cm and produced leaves. Leaves stayed on the explants until the 15th day after inoculation and then fell off. So, three experiments were conducted in an attempt to prevent leaf abscission by improving the culture vessel atmosphere.
Effect of silver nitrate and activated charcoal on Spanish cedar. Neither activated charcoal nor AgNO3 alone or combined prevented abscission of new foliage from Spanish cedar nodal explants (data not shown, F3, 16 = 1.12, P = 0.3). Ozudogrl et al. (2005) demonstrated that AgNO3 improved in vitro culture of some herbaceous species. In the present experiment, although AgNO3 at 3 mg l-1 lacked a statistical significant effect, it tended to decrease contamination while increasing leaf abscission.
The effect of AgNO3 on explant contamination could have been due to Ag+, which has bactericidal activity (Kuvshinov et al. 1999). Kane (2000) suggested AgNO3 at 1% as a surface disinfectant for explants; although we used 0.0003% AgNO3, this lower concentration may have helped to prevent contamination of inoculated explants. The effect of AgNO3 increasing leaf abscission could be explained by a phytotoxic effect of Ag+, which is a heavy metal (Pua 1999). For Petunia hybrid, AgNO3 also inhibited shoot proliferation (Dimasi-Theriou et al. 1993).
Activated charcoal also helped to prevent leaf abscission, although treatments were statistically similar (data not shown). This positive effect could be explained because activated charcoal is typically an absorbent for gases such as ethylene (Pan and van Staden 1998). Therefore, activated charcoal could have improved the vessel atmosphere and the explant responses.
Use of vented caps for Mahogany. The types of caps used on cultures failed to affect contamination percentages (F1, 10 = 0.09, P = 0.77) and bud break (F1, 10 = 4.04, P = 0.11) of Mahogany nodal explants. Nevertheless, the culture atmosphere seemed to be improved since fewer contaminated nodal explants were in vessels covered with vented caps than in normal caps (33.3 ± 16.2% and 43.2 ± 20.8%, respectively). Also, bud break was over double for nodal explants established in vessels with vented caps compared to those with normal caps (52.7 ± 13.0% and 25.3 ± 4.4%, respectively). These results could be explained by a positive ventilation effect in the vessel atmosphere, specifically by the filter of vented caps that might have allowed the exchange of air without microbial contaminants entering the vessel.
During photoautotrophic micropropagation of Eucalyptus, ventilation improved growth and quality, as well as lowered the percentage of contamination due to the manipulation of environmental conditions inside the culture vessels (Zobayed et al. 2004). In this study, such conditions prevented leaf abscission from shoots about 2 cm in length at least up to day 20. Leaves remained on shoots up to day 35, and then they started to drop from the shoot.
Use of Micropore tape as sealant for Spanish cedar. The results for the type of sealant preventing leaf abscission were similar to results for the type of caps. Contamination was similar for vessels sealed with either Micropore tape or plastic wrap (the normal sealant) (F1, 10 = 1.57, P = 0.24), and bud break was also unaffected by the type of sealant (F1, 10 = 0.41, P = 0.54). Nodal explants in vessels sealed with Micropore tape, however, grew better than those in vessels with the normal sealant since the contamination percentage was lower (30.1% ± 11.0% compared to 50.0% ± 11.4%) and the bud break percentage was higher (52.8% ± 10.0% compared to 43.0% ± 11.5%). The lower contamination yet higher bud break percentages could be due to better control of humidity inside the container allowed by the Micropore sealant.
Micropore tape is a breathable paper tape that allows gas exchange but keeps the culture atmosphere free from airborne bacteria and contaminants (Burne 2006). Gaseous substances such as CO2 and ethylene in the headspace of cultured explants could affect their in vitro response (Demeester et al. 1995). Sealing the containers with closure materials that allow gas exchange may be essential for bud break. Therefore, Micropore tape could be combined with vented caps in order to get a better control of the in vitro atmosphere to improve responses by Spanish cedar and Mahogany nodal explants.
In conclusion, PPMTM at 2 ml l-1 or NaOCl at 2% added to the medium and combined with NaOCl at 15% for 20 min as surface sterilization decreased contamination for single nodal explants from both Spanish cedar and Mahogany. These disinfection treatments allowed the stablishment of nodal explants from mature Meliaceae plants as the first stage of in vitro propagation. For both species, the combination of vented caps and micropore tape as sealant might improve the in vitro response of nodal explants during the establishment phase of micropropagation.
Acknowledgements
The first author is thankful to Consejo Nacional de Ciencia y Tecnología (CONACYT) and the Colegio de Postgraduados en Ciencias Agrícolas, both from México, and the Department of Plant, Soil and Entomological Sciences at the University of Idaho for providing the financial support for this research.
Bibliography
1 Burne, C. 2006. Tissue culture for gene transfer. Farming Ahead. 172: 44-45. [ Links ]
2 Collado, R.; Barbon, R.; Agramonte, D.; Jiménez, F.; Pérez, M.; Gutiérrez, O.; Ramírez, D. 2004. Establecimiento in vitro de ápices y segmentos nodales de Swietenia macrophylla King. Revista de Biotecnología Vegetal. 4(3): 143-146. [ Links ]
3 Demeester, J. J.; Matthijs, D. G.; Pascat, B.; Debergh, P. C. 1995. Toward a controllable headspace composition - growth, development, and headspace of a micropropagated Prunus rootstock in different containers. In Vitro Cellular and Developmental Biology. 31: 105-112. [ Links ]
4 Dimasi-Theriou, K.; Economou, A. S.; Sfakiotakis, E. M. 1993. Promotion of petunia (Petunia hybrida L.) regeneration in vitro by ethylene. Plant Cell Tissue and Organ Culture. 32: 219-225. [ Links ]
5 Fisse, J.; Batalle, A.; Pera, J. 1988. Endogenous bacteria elimination in ornamental plants. Acta Horticulturae. 212: 87-90. [ Links ]
6 Flores, C. A. 2001. Establecimiento de las etapas iniciales de la micropropagación de caoba (Swietenia macrophylla King) a partir de microestacas tomadas de plantas de invernadero. M. Sc. Thesis, CATIE, Turrialba, Costa Rica. 71 p. [ Links ]
7 Fuentes, S. R. L.; Calheiros, M. B. P.; Manetti-Filho, J.; Vieira, L. G. E. 2000. The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tissue and Organ Culture. 60: 5-13. [ Links ]
8 Guri, A. 1998. PPMTM - Plant Preservative Mixture. Plant Cell Technology, Inc. http:/www.ppm4plant-tc.com. Cited 30 April 2004 Guri, A. Z.; Patel, K. N. Compositions and methods to prevent microbial contamination of plant tissue culture media. United States Patent 5,750,402. [ Links ]
9 Jiménez, V. M.; Castillo, J.; Tavares, E.; Guevara, E.; Montiel, M. 2006. In vitro propagation of the neotropical giant bamboo, Guadua angustifolia Kunth, through axillary shoot proliferation. Plant Cell Tissue and Organ Culture. 86: 389-395. [ Links ]
10 Kane, M. 2000. Propagation from preexisting meristems. In: Trigiano, R. N.; Gray, D. J. eds. Plant Tissue Culture Concepts and Laboratory Exercises. CRC Press, Boca Raton, FL. 75-86. [ Links ]
11 Kitaya Y., Ohmura Y., Kubota C., Kozai T. 2005. Manipulation of the culture environment on in vitro air movement and its impact on plantlets photosynthesis. Plant Cell Tissue and Organ Culture. 83: 251-257. [ Links ]
12 Krikorian, A. D. 1994. Hormones in tissue culture and micropropagation. In: Davies, P. J.; ed. Plant Hormones, Physiology, Biochemistry and Molecular Biology. Dordrecht: Kluwer Academic Publishers. 774-796. [ Links ]
13 Kuvshinov, V.; Koivu, K.; Kanerva, A.; Pehu, E.1999. Agrobacterium tumefasciens-mediated transformation of greenhouse-grown Brassica rapa ssp. oleifera. Plant Cell Reports. 18: 773-777. [ Links ]
14 Lee, S. K.; Rao, A. N. 1988. Plantlet production of Swietenia macrophylla King through tissue culture. Gardening Bulletin Singapure. 41: 11-18. [ Links ]
15 Leifert, C.; Waites, W. M. 1992. Bacterial growth in plant tissue culture media. J. Appl. Bacteriol. 72: 460-466. [ Links ]
16 Maruyama, E. 2006. Tissue culture of Swietenia macrophylla King (Big-Leaf Mahogany). In: Suzuki, K.; Ishii, K.; Sakurai, S.; Sasaki, S.; eds. Plantation Technology in Tropical Forest Science. Springer-Verlag Tokyo. 131-136. [ Links ]
17 Maruyama, E.; Ishii, K.; Saito, A.; Migita, K. 1989. Screening of suitable sterilization of explants and proper media for tissue culture of eleven tree species of Perú - Amazon forest. Journal of Agricultural Science (Japan). 33: 252-260. [ Links ]
18 Mroginski, E.; Rey, H. I.; Mroginski, A. 2003. In vitro plantlet regeneration from Australian Red Cedar (Toona ciliata, Meliaceae). New Forests. 25: 177-184. [ Links ]
19 Murashige, T.; Skoog, F. 1962. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologia Plantarum. 15: 473-497. [ Links ]
20 Niedz, R. P. 1998. Using Isothiazolone biocides to control microbial and fungal contaminants in plant tissue cultures. HortTechnology. 8: 598-601. [ Links ]
21 Ozudogrl, E. A.; Ozden-Tokatli, Y.; Akcin, A. 2005. Effect of silver nitrate on multiple shoot formation of Virginia-type peanut through shoot tip culture. In Vitro Cellular and Developmental Biology Plant 41: 151-156. [ Links ]
22 Pan, M. J.; van Staden, J. 1998. The use of charcoal in in vitro culture - A review. Plant Growth Regulators. 26: 155-163. [ Links ]
23 Pérez, J.; Mesén, F.; Hilje, L.; Aguilar, M. E. 2006. Método de micropropagación aplicable a genotipos selectos de Cedrela odorata. Fases de desarrollo y enraizamiento. Recursos Naturales y Ambiente. 46-47: 146-151. [ Links ]
24 Pua, E. C. 1999. Morphogenesis in cell and tissue cultures: role of ethylene and polyamines. In: Soh, W. Y.; Bhojwani, S. S., eds. Morphogenesis in plant tissue cultures. Dordrecht: Kluwer Academic Publishers. Pp. 255-303. [ Links ]
25 Rodríguez, R.; Daquinta, M.; Capota, I.; Pina, D.; Lezcano, Y.; González-Olmedo, J. L. 2003. Nuevos aportes a la micropropagación de Swietenia macrophylla x Swietenia mahogani (Caoba Híbrida) y Cedrela odorata (Cedro). Cultivos Tropicales. 24: 23-27. [ Links ]
26 Salas, S. A. 2000. Resumen acumulado de datos agroclimáticos. CATIE. Turrialba, Costa Rica. [ Links ]
27 SAS Institute Inc. SAS for windows. 9th edn. Cary, North Carolina, USA. 2001. [ Links ]
28 Schenk, R. V.; Hildebrandt, A. C. 1972. Medium and techniques for induction and growth of monocotyledoneous and dicotyledoneous plant cell cultures. Canadian Journal of Botany. 50: 199- 204. [ Links ]
29 Sharma, S. K.; Ramamurthy, V. 2000. Micropropagation of 4-year-old elite Eucalyptus tereticornis trees. Plant Cell Reports. 19: 511-518. [ Links ]
30 Teixeira, S. L.; Ribeiro, J. M.; Teixeira, M. T. 2006. Influence of NaOCl on nutrient medium sterilization and on pineapple (Ananas comosus cv. Smooth Cayenne) behavior. Plant Cell Tissue and Organ Culture. 86: 375-378. [ Links ]
31 Thakur, R.; Sood, A. 2006. An efficient method for explant sterilization for reduced contamination. Plant Cell Tissue and Organ Culture. 84: 369-371. [ Links ]
32 Zobayed, S. M. A.; Afreen, F.; Xiao, Y.; Kozai, T. 2004. Recent advancement in research on photoautotrophic micropropagation using large culture vessels with forced ventilation. In Vitro Cellular and Developmental Biology Plant. 40: 450-458. [ Links ]