Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Biotecnología
Print version ISSN 0123-3475
Rev. colomb. biotecnol vol.16 no.1 Bogotá Jan./June 2014
https://doi.org/10.15446/rev.colomb.biote.v16n1.40495
http://dx.doi.org/10.15446/rev.colomb.biote.v16n1.40495
ARTÍCULO DE INVESTIGACIÓN
Identification, cloning and lactonase activity of recombinant protein of N-acyl homoserine lactonase (AiiA) from Bacillus thuringiensis 147-115-16 strain.
Identificación, clonación y actividad lactonasa de la proteína recombinante de N-ácil homoserina lactonasa (AiiA) de Bacillus thuringiensis cepa 147-115-16
Short title: N-acyl homoserine lactonase (AiiA) from Bacillus thuringiensis
Alvaro M. Florez1, Adriana Gonzalez2, Carmen J. Pedroza2, Elizabeth Correa2, Nohora J. Rueda2 y Sergio Orduz2.
1 Laboratorio de Biolología Molecular y Biotecnología, Facultad de Medicina, Universidad de Santander, UDES, Bucaramanga, Santander, Colombia.
2 Escuela de Biociencias, Facultad de Ciencias, Universidad Nacional de Colombia sede Medellin, Medellin, Colombia.
Correspondence author amflorez@udes.edu.co. Phone +57 6516500 Ext. 253. Fax: +57 6516492
Recibido: octubre 25 de 2013 Aprobado: mayo de 2014
Abstract
The quorum-quenching N-acyl homoserine lactonases are a family of bacterial metalloenzymes that participate in degradation of N-acyl homoserine lactones (AHLs), disrupting the quorum sensing system of gram negative bacterial species. From a collection of Bacillus thuringiensis strains isolated in Colombia from plants and exhibiting toxic activity against lepidopteran insects, 310 bacterial isolates were tested to determine lactonase activity by using biosensor systems in presence of synthetic N-hexanoyl-L-homoserine lactone (C6-HSL) and N-octanoyl-L-homoserine lactone (C8-HSL). From them, 251 strains showed degrading activity to both C6-HSL and C8-HSL, 57% exhibited degrading activity to C6-HSL and 43% to C8-HSL. One B. thuringiensis strain, denoted as 147-115-16, that exhibit high degrading activity to C6-HSL and C8-HSL, was able to attenuate soft rot symptoms in infected potato slices with Pectobacterium carotovorum. This strain contains an homologous of the aiiA gene that was cloned, sequenced and expressed in Esherichia coli DE3. The recombinant protein AiiA147-11516 display activity to C6-HSL, C8-HSL, N-(β-ketocaproyl) (3-O-C6-HSL) and N-3-oxo-dodecanoyl (3-O-C12-HSL). The recombinant strain in the presence of P. caratovorum cultures was able to attenuate the infection, suggesting that it interferes either on the accumulation or response to the AHLs signals. Acording to this data and based on previous report from recombinant AiiA147-11516, this enzyme exhibit activity to wide range of catalytic substrates suggesting its industrial application in the disease control programs by plants transformation.
Key words : lactones, Quorum sensing, Quorum quenching, Lactonases, Pectobacterium caratovorum
Resumen
Las N-acíl homoserina lactonasas son una familia de metaloenzimas bacterianas que participan en la degradación de N-acil homoserina lactonas (AHLs) interrumpiendo el sistema de detección de quórum sensing de bacterias Gram negativas. A partir de una colección de cepas de Bacillus thuringiensis aisladas del filoplano de plantas colombianas que presentaron actividad tóxina contra insectos lepidópteros, 310 fueron probadas para determinar actividad lactonasa mediante el uso de sistemas de biosensores en presencia de N-hexanoilo-L-homoserina lactona (C6-HSL) y la N-octanoilo-L-homoserina lactona (C8-HSL) sintéticas. De estas cepas, el 251 mostraron actividad para ambas lactonas y de estas, el 57% mostró actividad a C6-HSL y el 43% a C8-HSL. Una cepa de B. thuringiensis- denominada 147-115-16- que mostró alta actividad para C6-HSL y C8-HSL, fue capaz de atenuar los síntomas de la pudrición blanda en rodajas de papa infectadas con Pectobacterium carotovorum. Esta cepa contiene un gen homólogo a aiiA, el cual este fue clonado, secuenciado y expresado en Escherichia coli DE3. La proteína recombinante AiiA147-11516 exhibe actividad para C6-HSL y C8-HSL, así como para N-(β-cetocaproil) (3-O-C6-HSL) y N-3-oxo-dodecanoil (3-O-C12-HSL). La cepa recombinante en presencia de P. caratovorum fue capaz de atenuar la infección, sugiriendo que interfiere con la acumulación o respuesta de las señales AHLs. Según estos datos y basados en el reporte previo sobre la actividad hidrolítica de la proteína recombinante AiiA147-11516, esta enzima posee un actividad contra un amplio número de sustratos lo cual sugiere su aplicación en la industria en el control de enfermedades, mediante la transformación de plantas.
Palabras Clave : Lactones, Quorum sensing, Quorum quenching, Lactonases, Pectobacterium caratovorum.
Introduction
In the majority of gram negative bacteria quorum sensing is mainly mediated by signaling cascades, involving N-acyl-homoserine lactones (AHLs) (Von Bodman et al., 2003). AHLs contain a common homoserine lactone ring, differing in their carbon lenght (4 to 18 carbons), degree of saturation, and the presence of C3 acyl chain modifications. AHLs are multifunctional molecules that when they accumulate regulate relevant functions during bacterial growth such as production of extracellular enzymes, bioluminescence, biofilm formation, and coordinate cellular activities associated with host pathogen interactions (Atkinson and Williams, 2009; Williams and Camara, 2009). Many bacterial species, such as Pectobacterium carotovorum, are highly pathogenic to plants by triggering the expression of virulence factors via quorum sensing (QS) (Williams, 2007). P. caratovorum is an ethiologic agent of soft rot disease in a wide variety of cultivated plants (Charkowski, 2009). This bacterium regulates the production of different exoenzymes by secreting AHLs, including N-(3-oxohexanoyl)-L-homoserine lactone (3-O-C6-HSL) and N-(3-oxooctanoyl)-L-homoserine lactone (3-O-C8-HSL) (Jones et al., 1993; Pirhonen et al., 1993; Cha et al., 1998; Smadja et al., 2004; Chatterjee et al., 2005; Liu et al., 2008). The role of QS in P. caratovorum pathogenecity have been reviewed (Barnard et al., 2007).
In contrast, there are microorganims acting as antagonists of QS by producing signal interference mechanisms known as quorum quenching (QQ), (Kalia, 2013) mediated by AHL-degrading enzymes. There are two families of QQ enzymes, lactonases and acylases. The lactonase family cleaves the lactone ring of AHLs releasing N-acyl homoserines, which are not recognized as a QS signal, some examples include AiiA from Bacillus sp., AttM from Agrobacterium tumefaciens strain A60, AiiB from A. tumefaciens strain C58 (Carlier et al., 2003; Dong et al., 2000) and AhlD from Arthrobacter sp. (Park et al., 2003). Acylases are known to be capable of cleaving the acyl bonds and releasing a homoserine lactone ring (Lin et al., 2003; Sio et al., 2006), some representative enzymes are AiiD from Ralstonia XJ12B, and PvdQ from Pseudomonas aeruginosa PAO1. After the cleavage of the AHLs molecules, the released products become more soluble in water, making them an available nutrient source to the sorrounding microorganisms, like Variovorax paradoxus (VAI-C), which is capable of using AHLs as a sole nutrient source (Leadbetter and Greenberg, 2000).
Production of QQ N-acyl homoserine-lactonases was first reported in a strain of the genus Bacillus sp. (Dong et al., 2000; Dong et al., 2004). It was also reported in Bacillus thuringiensis, B. cereus and B. mycoides (Dong et al., 2002; Lee et al., 2002). The first AHL-lactonase that was completely charaterized was AiiA240B1 and it was found hat belongs to the metallo-β-lactamase family based on the presence of a HXHXDH motif, and an identical Zn2+ binding motif (Dong et al., 2000). This enzyme has the ability to inactivate AHLs with different acyl chain lengths and substitutions at the C3 position, including N-butyryl-L-homoserine lactone (C4-HSL), 3-O-C6-HSL, 3-O-C8-HSL, and 3-O-C12-HSL (Dong et al., 2001).
The second AHL-lactonase (AiiABTK) reported was isolated from B. thuringiensis subsp. kurstaki, and work on a wider range of AHLs substrates, with maximum activity towards C10-HSL. This enzyme contains two Zn2+ ions into the active site and possesses the same conserved residues present in all AHL-lactonases whose activity depends in metal coordination (Kim et al., 2005). For both characterized enzymes, AiiA240B1 and AiiABTK, important differences in the affinity for metal ions under chelating agents have been described (Wang et al., 2004; Kim et al., 2005; Thomas et al., 2005).
Based on that there is widespread existence of AHL-lactonases in B. thuringiensis (Dong et al., 2002; Lee et al., 2002), and in Colombia more than 600 B. thuringiensis isolates have been characterized looking for insecticidal activity (Uribe et al., 2003; Jara et al., 2006; Armengol et al., 2007), we aimed at isolating AHL degrading from a B. thuringiensis collection obtained from Colombian phyloplane. In this study we screened a B. thuringiensis collection and described the cloning, sequencing and the activity of recombinant protein of a N-acyl homoserine lactonase from B. thuringiensis 147-11516 .
Materials and methods
Bacterial strains and growth conditions
Two different AHL biosensors strains were used to detect AHL-lactonase activity, Chromobacterium violaceum CVO26 (mini Tn5 mutant of 31532) (McClean et al., 1997; Winson et al., 1998) and A. tumefaciens NTL4 traI-lacZ fusion (pCF218) (pCF372) (Fuqua et al., 1994). C. violaceum CVO26 was grown in LB medium (1% peptone, 0.5% yeast extract, 0.5% NaCl) solidified with 1.2% agar, and supplemented with kanamycin (20 µg/ml) (Invitrogen®). A. tumefaciens NTL4 was grown in AT minimal salts medium as was described in Tempé et al. (1977), at 30ºC, supplemented with tetracycline 4.5 µg/ml (Sigma-Aldrich®), spectinomycin 50 µg/ml (Sigma-Aldrich®) and 40 µg/ml of X-gal (Promega®). Escherichia coli ArticExpress (DE3) cells were used for cloning and expression and were cultured at 37ºC in LB medium supplemented with 100 µg/ml of ampicillin (Sigma-Aldrich®). B. thuringiensis strains were cultured in LB medium at 30ºC in agar plates and broth.
Plant material with symptoms of soft rot disease were selected in order to isolate bacterial plant pathogens as described (Pérombelon and Kelman, 1980). Samples were taken from affected zones from lettuce (Lactuca sativa var. Batavia), calla lily (Zantedeschia aethiopica var. Spreng), potato (Solanum tuberosum var. Diacol capiro) and lulo (S. quitoense var. Castilla), and processed according to phytopathological methods (Agrios, 2005). Different bacterial colonies were obtained in nutrient agar from serial dilutions of macerated tissues. The isolated colonies were grown in D-3 selective medium for P. caratovorum (Kado et al., 1970). Complementary tests for species identification were carried out as described (Schaad et al., 2001).
B. thuringiensis AHL-lactonase activity
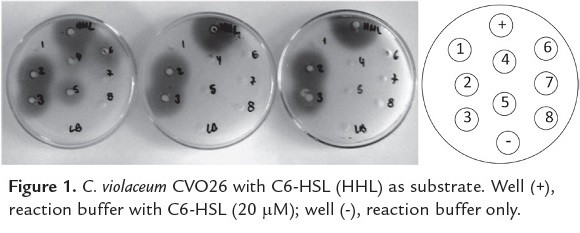
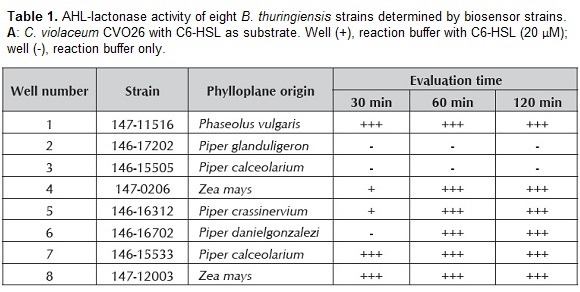
AHL-lactonase activity was evaluated in 310 B. thuringiensis isolates as described by Lee et al. (2002), with some modifications. A culture of each B. thuringiensis strain was grown overnight until reaching an optical density of 1.1 at 600 nm. Then, 20 µM of synthetic C6-HSL and C8-HSL (Sigma-Aldrich®) were added independently into each culture and the reaction mixture was incubated at 30ºC with shaking during 30, 60 and 120 min. Bacterial cells were centrifuged at 3000 x g for 10 min and the supernatants corresponding to each incubation time and to each B. thuringiensis strain were heated for 3 min at 95ºC and placed into petri dishes with 10 wells with LB medium or AT agar containing an agar-suspension culture of the biosensor strain. These plates were incubated for 24 h at 30ºC. The AHL-lactonase activity was evaluated at different times by measuring the diameters of the colored areas surrounding the wells in CVO26 (purple) and NTL4 (blue). Based on the sizes of the colored areas, a scale was designed; diameters between 0.0 and 0.39 cm2 were assigned with (+++) indicating B. thuringiensis strains displaying high AHL-lactonase activity; between 0.4 and 0.74 cm2 were assigned with (++) indicating a moderate activity; and between 0.75 and 0.89 cm2 were assigned with (+) indicating a low activity. Finally, diameters larger than 0.9 cm2 were considered without AHL-lactonase activity. Only the B. thuringiensis strains that exhibit high AHL lactonase activity (+++) to each substrate to either C6-HSL or C8-HSL at 30, 60 and 120 min or 60 and 120 min were reported.
Screening of AHL production by P. caratovorum
The plant pathogenic bacterium P. caratovorum was streaked in parallel to the biosensor strains in LB agar for CVO26 and in the same way for NTL4 but in AT medium supplemented with 50 µg X-gal (Ravn et al., 2001; Chu et al., 2011), and plates were incubated for 24 h at 30ºC. To evaluate the presence of AHLs, CVO26 was streaked on LB agar supplemented with C6-HSL, because its sensor system is inhibited by long acyl chain molecules. For this last assay, all plates with CVO26 were incubated for 24 h at 30ºC for production of purple pigment, and then plates were re-incubated at 30ºC for 24 h in order to evaluate inhibition of pigment formation.
In vitro control of soft rot symptoms
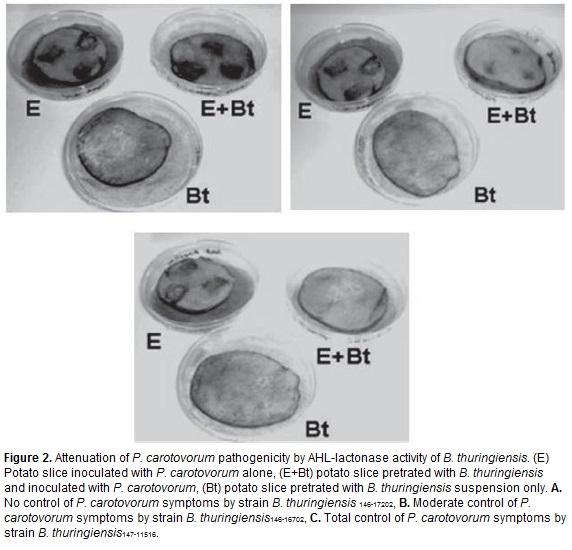
To evaluate the effect of AHL-lactonase activity of B. thuringiensis on P. caratovorum infection, potatoes (S. tuberosum var Diacol Capiro) were washed with tap water and treated as described Dong et al. (2004). Five mm thick potato slices were dipped in a B. thuringiensis 12 hr culture containing 2x107 CFU/ml for 20 s and then were air dried in a laminar flow cabinet for 20 min. Then, three streaks were made on the surface of the slices and inoculated with 2.5 µl of cell culture media containing 1x105 UFC/ml of an P. caratovorum suspension isolated from potato. Each slice was placed in a petri dish in a humid chamber at 30ºC. Disease symtoms were evaluated after 24 and 48 h of treatment. Each treatment was performed in quadruplicate. Based on the severity of the soft rot symptoms produced in the potato slices, a scale was developed to qualify the effect of B. thuringiensis on the disease caused by P. caratovorum. Absence of soft rot was assigned with (-), presence of small soft rot area and little wet was considered with (+), presence of moderate soft rot area, dark and wet was considered with (++) and, presence of high soft rot area, very dark and very wet was considered with (+++).
Cloning and sequencing of the aiiA gene
The B. thuringiensis strain displaying greater AHL-lactonase activity with the biosensor strains A. tumefaciens NTL4 and C. violaceum CVO26 and the best control of P. caratovorum pathogenicity was selected and its DNA was extracted (Ceron et al., 1995). Using PCR the aiiA gene was amplified with the following primers: 5´ATCGGATCCATGACAGTAAAGAAGCTTTATTTCG3´and 5´GTCGAATTCCTCAACAAGATACTCCTAATGATGT3´ designed according to the aiiA sequences previously reported (Dong et al., 2002). Amplification conditions were: denaturation at 94ºC for 3 min followed by 28 cycles at 94ºC for 30 s, 50ºC for 30 s, and 72ºC for 1 min in a MJ Research Thermocycler (Bio-Rad®). The amplified fragments were purified with a QIAquick PCR purification system (Qiagen®) and cloned into pGEM-T vector (Promega®). Clones were selected in LB media supplemented with IPTG (40 µg/ml) (Invitrogen®), X-gal (40 µg/ml) (Invitrogen®) and ampicillin (100 µg/ml) (Sigma-Aldrich®). The gene was sequenced in both strands and the results were analyzed using Blastn, Blastp and ClustalW algorithm in Bioedit (Hall, 1999). The similarity and identity analyses were performed using MatGat (Campanella et al., 2003), and evolutionary relationships between AHL-lactonases were done using MEGA5 (Tamura et al., 2011).
The subcloning into the expression vector pCold IV (Takara- BIO-INC ®), gene expression and protein purification are described by Pedroza et al. (2014).
Homology and phylogenetic dendrogram of AHL-degrading enzyme
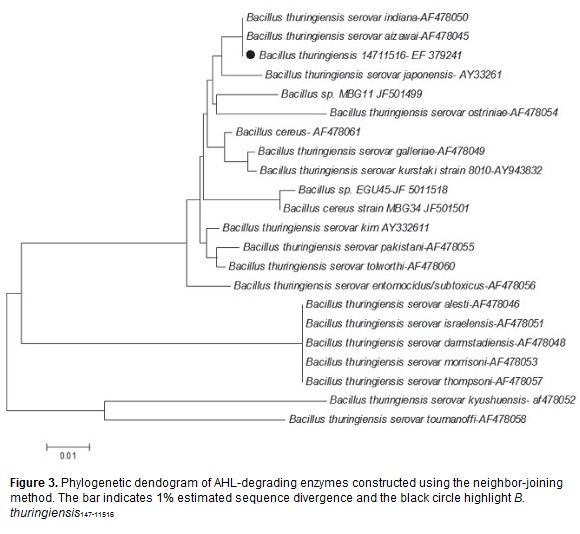
The similarity and identity analyses were performed using MatGat (Campanella et al., 2003), and evolutionary relationships between AHL-lactonases were done using MEGA5 (Tamura et al., 2011). The evolutionary history was inferred using the Neighbor-Joining Method (Saitou and Nei, 1987). The optimal tree with the sum of branch length was = 0.34721705. The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood Method (Tamura et al., 2004) and are in the units of the number of base substitutions per site. The analysis involved 22 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 570 positions in the final dataset.
AHL-lactonase activity of recombinant E. coli DE3
The recombinant E. coli DE3 containing the expression cassette pColdIV-aiiA was grown at 37ºC in LB broth with ampicillin (50 µg/ml) at 200 rpm. After 5 hours of growth (O.D. 600 nm = 0.83), the IPTG (3.2 mM) was added and the incubation was continued overnight to reach an O.D. of 2.6 at 600 nm (Zhang et al., 2007). The cell culture was divided in several fractions in order to determine the AHL-lactonase activity. The first and second fractions were added each to LB medium in presence of 100µ M of synthetic C8-HSL and C6-HSL, the third and fourth fractions were added to LB medium in the presence of 100 µM of synthetic 3-O-C6-HSL and 3-O-C12-HSL, respectively. The fifth fraction was co-inoculated with 20 µl of a P. caratovorum culture (OD600 = 0.8) in 5 ml of LB media. All the fractions were incubated at 37ºC for 18 h at 200 rpm. The experiments included B. thuringiensis 147-11516, as positive control and E. coli DE3 strain and P. caratovorum alone as negative control. Once the incubation time was finished each culture was centrifuged at 5800 x g during 10 min at 21ºC and the supernatants were collected. Six lines were made with a sterile blade on each petri dish containing either LB media for CV026 strain or AB media with X-gal for NTL4 strain. Each line was 1 cm wide and 3 mm apart. Five µL of each supernatant was served in one end of each groove, and the samples were allowed to dry, and then each respective reporter strain was streaked. The petri dishes were incubated at 30ºC for 24 hours. To determine the pigment produced by the reporter strains, the length was measured and the reproducibility was determined by 20 replicates.
Statistical Analysis
Data was analyzed by using the ANOVA test. The confident values were set at 95%.
Results
B. thuringiensis AHL-inactivating activity
Inactivation of AHLs by B. thuringiensis strains is considered effective when in a given evaluation period, the size of the colored area decreases with the time. When no color is observed at the first time of evaluation (30 min), the strain is listed as a candidate for in vitro pathogenicity inhibition assays. From the 310 B. thuringiensis strains isolated from phylloplane plants tested, 251 showed AHL-lactonase activity with C6-HSL and C8-HSL; C6-HSL was degraded by 57% of the B. thuringiensis strains, while C8-HSL was inactivated by 43% of the B. thuringiensis strains. B. thuringiensis strains that exhibit strong AHL lactonase activity against C6-HSL and C8-HSL are shown in table 1. The screening to these strains using CV026 in are shown in figure 1. The detectable concentration of the reporter strains was between 100-120 µM of AHLs.
Effect of B. thuringiensis cultures on soft rot symptoms caused by P. caratovorum.
To test the attenuation of soft rot symptoms, B. thuringiensis strains displaying the highest AHL-lactonase activity according to the results with the biosensor strains were selected. The plant pathogen P. caratovorum isolated from potato was used in these assays and its pathogenicity was evaluated on potato slices showing severe tissue maceration. When potato slices were pretreated with B. thuringiensis suspension (2x107 UFC/ml) before inoculation with P. caratovorum, the soft rot symptoms were considerably less severe compared with the soft rot produced on slices without previous treatment. According to the evaluation scale of soft rot symptoms, the strain B. thuringiensis147-115-16 displayed the highest efficiency inhibiting P. caratovorum pathogenicity (figure 2).
Cloning and sequence analysis of the aiiA gene
B. thuringiensis147-11516 strain displayed the best results in the biosensor assays, as well as in the in vitro pathogenicity assays; therefore, it was selected for molecular characterization. The length of expected amplified PCR product containing the gene coding aiiA was around 820 bp. Several clones that showed this length, exhibit a 754 bp ORF with sequence homology to aiiA genes reported in GenBank. The DNA sequence of B. thuringiensis 147-11516 gene was reported in GenBank with accession number EF379241. Comparison of the B. thuringiensis147-11516 aiiA gene with other aiiA genes revealed maximum identity in the nucleic acid sequence between 93% to 99%. The dendogram show that aiiA gene of B. thuringiensis147-11516 is most similar to B. thuringiensis subsp.aizawai and indiana (figure 3). At protein level three conserved domains in the protein aiiA of the B. thuringiensis147-11516 were detected by BLAST CD search of Conserved Domain Database (rpsblast; http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi; domain COG0491GloB, Zn dependent hydrolases, including glyoxylases (residues 78-250); domain pfam00753, the lactamase-β domain, metallo-beta-lactamase superfamily (residues 33-235); and domain COG1237, metal dependent hydrolases domain of the β lactamase superfamily (residues 22-161). Using MEME and MAST programs (Bailey and Gribskov, 1998), in which protein sequences are analyzed for similarities among hydrolases, three motifs were also found in B. thuringiensis 147-11516 aiiA: motif 1 (residues 11-60); motif 2 (residues 76-125), and motif 3 (residues 128-177). These motifs of the aiiA are also present in the three Zn-dependent hydrolases.
Recombinant E. coli DE3- pCold-aiiA shown lactonase activity
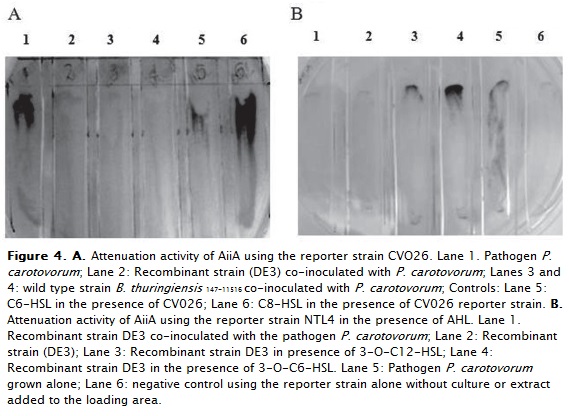
The lactonase activity of the recombinant E. coli DE3 was tested with synthetic lactones, C8-HSL and C6-HSL in the presence of the reporter strains CV026 and, with 3-O-C6-HSL and 3-O-C12-HSL in the case of NTL4. The results showed lack of purple pigmentation of strain CV026 (figure 4) and in the case of NTL4, exhibit lack of blue pigmentation. In the presence of the pathogen P. caratovorum, the QQ signals were disrupted (figure 4A, lane 2 and 4B lane 1).
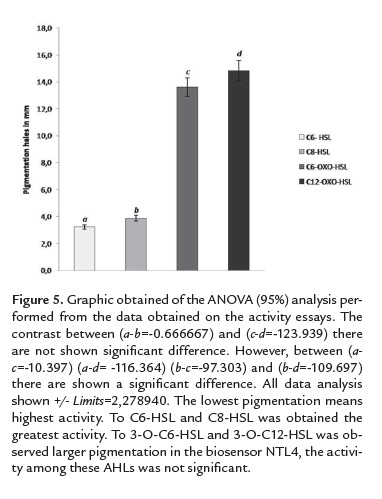
In all the cases, disruption of QS by the recombinant E. coli DE3, is shown by the lack of pigmentation in the presence of biosensor strains in comparison with the control, with the pathogen alone, which exhibits pigment production in response to both, long and short chain lactones. ANOVA analysis showed statistically significant differences between lactones indicating the preference to short chain than those that exhibit long-chain oxo substitutions. There was no statistical difference within the same group of lactones (figure 5). These results demonstrated the preference of recombinant AiiA147-11516 for C8-HSL and C6-HSL.
Discussion
N-acyl homoserine lactones are signaling molecules that are inactivated by the enzyme AHL-lactonase by hydrolyzing the lactone ring, and in consequence, inhibiting the virulence of gram negative pathogens such as P. caratovorum (Dong et al., 2004). In this study, the strain B. thuringiensis 147-11516 isolated from the phylloplane of Colombian plants, was found to display attenuation of P. caratovorum symptoms in potato bioassays.
It has been established that the potential of some B. thuringiensis strains to attenuate infections depends on breaking down AHL molecules that contain different acyl side chain lengths (Lee et al., 2002). In this study, two synthetic AHLs with different acyl chain lengths, C6-HSL and C8-HSL, were inactivated by B. thuringiensis 147-11516 detected by the biosensor strains A. tumefaciens NTL4 and C. violaceum CV026. The biosensor strain NTL4 showed blue color as response in the presence of AHLs produced by P. caratovorum. In the in vitro assays using pretreated potatoes slices with B. thuringiensis 147-11516 cultures, attenuation of the soft rot symptoms caused by P. caratovorum were observed, which could suggest an interference of the B. thuringiensis 147-11516 AiiA towards AHL signaling molecules produced by P. caratovorum. This signaling molecules including those that have been reported to P. caratovorum such as 3-oxo substitutions in the acyl chain (Chatterjee et al., 2005), have been reported as a main regulators of extracellular enzymes involved in potato infection and responsible for symptoms development (Jones et al., 1993; Pirhonen et al., 1993). The B. thuringiensis 147-11516 strain contains also the aiiA gene that encodes a AiiA protein with a molecular weight of approximately 28,2 kDa, which is similar to the predicted molecular mass of 28,036 Da of the protein reported by Dong et al. (2000). The amino acid sequence alignments showed identities of 90.8% to 99.6% to other AHL-lactonases described (Lee et al., 2002). The evolutionary distances used to infer a phylogenetic tree confirm the wide distribution of aiiA homologous genes in B. thuringiensis subspecies and individuals and with identities above 93%. At protein level it was also observed the region 104HLHFDH109≈ H169, that correspond to the motif 104HXHXDH109≈ H169 which is a complety conserved region among AiiA proteins. This motif contains a dinuclear Zn2+ center in which the Zn1 is coordinated by H104, H106 and H169 while the Zn2 is coordinated by H109, H235, D108 and D191 (Kim et al., 2005). According to this data, the AiiA from B. thuringiensis 147-11516 seems to be metallo hydrolytic enzyme. The differences observed between AiiA from B. thuringiensis 147-11516 (Pedroza et al., 2014) and AHL- lactonaseBTK-AiiA (Kim et al., 2005) in 23 amino acids in which Ile-73 and Met-138 are part of hydrophobic channel and Tyr-165 and Ser-170 differ from the conserved region 165HTPGHTPGH173 involved in Zn coordination, could explain the capability of AiiA147-11516 to retain the activity in presence of high concentrations of EDTA and increase three and four times its activity to C4-HSL and C8-HSL respectively (Pedroza et al., 2014).
The lactonase activity of the recombinant strain DE3 harboring the construct pCOLDIV:aiiA was evaluated in the presence of synthetic lactones with different acyl side chain lengths, C6-HSL, C8-HSL, 3-O-C6-HSL and 3-O-C12-HSL in the presence of the reporter strains C. violaceum CV026 and A. tumefaciens NTL4. Based on the observation that the lactones are diffusible on solid media, and that the lactonase activity is inversely proportional to the color intensity, a light blue pigment of the biosensor was observed to 3-O-C6-HSL and 3-O-C12-HSL when they were tested with the biosensor strain NTL4. These data indicates that lactonase inactivation was not complete and that there was remaining lactones in the media (data not shown). It was also observed the ability of recombinant E. coli DE3 strain to disrupt P. caratovorum communication when they were co-inoculated in the presence of the reporter strain. This attenuation suggests that the lactonase produced by the recombinant strain exhibit activity to modified lactones and interferes to signaling molecules used by P. caratovorum.
The high homology and the presence of one conserved motif 104HXHXDH109≈ H169 indicate that the AHL-lactonase147-11516 belongs to the AHL-lactonase family. The ability of the recombinant strain in P. caratovorum suggest an important role of AHL- lactonase to interfer the AHLs signaling or AHLs response. Recently, we report the enzymatic hydrolysis from recombinant AiiA147-11516 lactonase in order to determine its application (Pedroza et al., 2014). The activity to broad catalytic spectrum of lactones including as N-butyryl-L-Homoserine lactone (C4-HSL), N-heptanoyl-L-Homoserine lactone (C7-HSL) and the enzymatic hydrolysis to C6-HSL in alkaline pH, activity in high temperature (60°C) and decreasing of enzymatic activity by two metal ions, suggest that this enzyme is a promissory candidate for the disease control programs by plants transformation. Other escenarios such as the addition of enrichment cultures of AHL degrading bacteria Bacillus sp. that increase the survival of aquaculture species (Tinh et al., 2008), the control over QS based bacterial biofilms in biorefineries (Hong et al., 2012) and the incoporation of QS inhibitors in medical and dental implants and catheters (Choudhary and Schmit-Dannert, 2010), provide further applications.
Aknowledgments
The authors thank Dr. Clay Fuqua (Indiana University, Bloomington, Indiana) for providing C. violaceum CVO26 and A. tumefaciens NTL4. We thank Dr. Oscar Alzate, for his critical review of the manuscript. This work was supported by grants from The Colombian Department of Science, Technology and Innovation (2213-12-13773 and 1299-12-17827) to AMF.
Bibliography
1 Armengol G., Escobar M.C., Maldonado M.E., Orduz S. 2007. Diversity of Colombian strains of Bacillus thuringiensis with insecticidal activity against dipteran and lepidopteran insects. Journal of applied microbiology. 102(1): 77-88. [ Links ]
2 Atkinson S., Williams P. 2009. Quorum sensing and social networking in the microbial world. Journal of The Royal Society Interface. 6(40): 959-678. [ Links ]
3 Bailey T.L., Gribskov M. 1998. Methods and statistics for combining motif match scores. Journal of Computational Biology. 5(2): 211-221. [ Links ]
4 Barnard A.M., Bowden S.D., Burr T.S., Coulthurst J., Monson R.E., Salmond G.P. 2007. Quorum sensing, virulence and secondary metabolite production in plant soft-rotting bacteria. Philosophical Transactions of the Royal Society B: Biological Sciences. 362(1483): 1165-1183. [ Links ]
5 Campanella J.J., Bitincka L., Smalley J. 2003. MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics. 4(1): 29. [ Links ]
6 Carlier A., Uroz S., Smadja B., Fray R., Latour X., Dessaux Y., Faure D. 2003. The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-Acyl homoserine lactonase activity. Applied and Environmental Microbiology. 69(8): 4989-4993. [ Links ]
7 Cerón J., Ortiz A., Quintero R., Guereca L., Bravo A. 1995. Specific PCR primers directed to identify cryI and cryIII genes within a Bacillus thuringiensis strain collection. Applied and Environmental Microbiology. 61(11): 3826-3831. [ Links ]
8 Cha C., Gao P., Chen Y.C., Shaw P.D., Farrand S.K. 1998. Production of acyl-homoserine lactone quorum-sensing signals by gram negative plant-associated bacteria. Molecular Plant-Microbe Interactions. 11(11): 1119-1129. [ Links ]
9 Charkowski A.O. 2009. Decaying signals: will understanding bacterial-plant communications lead to control of soft rot?. Current opinion in Biotechnology. 20(2): 178-184. [ Links ]
10 Chatterjee A., Cui Y., Hasegawa H., Leigh N., Dixit V., Chatterjee A.K. 2005. Comparative analysis of two classes of quorum-sensing signaling systems that control production of extracellular proteins and secondary metabolites in Erwinia carotovora subspecies. Journal of Bacteriology. 187(23): 8026-8038. [ Links ]
11 Choudhary S., Schmidt-Dannert C. 2010. Applications of quorum sensing in biotechnology. Applied Microbiology and Biotechnology. 86(5): 1267-1279. [ Links ]
12 Chu W., Vattem D.A., Maitin V., Barnes M.B., McLean R.J.C. 2011. Bioassays of Quorum Sensing Compounds using Agrobacterium tumefaciens and Chromobacterium violaceum. In: Quorum Sensing: Methods and Protocols, Methods in Molecular Biology. Rumbaugh, K.P. (Ed). Springer Science-Business Media. [ Links ]
13 Dong Y.H., Xu J.L., Li X.Z., Zhang L.H. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proceedings of the National Academy of Sciences. 97(7): 3526-3531. [ Links ]
14 Dong Y.H., Wang L.H., Xu J.L., Zhang H.B., Zhang X.F., Zhang L.H.2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 411(6839): 813-817. [ Links ]
15 Dong Y.H., Gusti A.R., Zhang Q.J., Xu L. Zhang L.H. 2002. Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Applied and Environmental Microbiology. 68(4): 1754-1759. [ Links ]
16 Dong Y.H., Zhang X.F., Xu J.L., Zhang L.H. 2004. Insecticidal Bacillus thuringiensis silences Erwinia carotovora virulence by a new form of microbial antagonism, signal interference. Applied and Environmental Microbiology. 70(2): 954-960. [ Links ]
17 Fuqua W.C., Winans S.C., Greenberg E.P. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. Journal of Bacteriology. 176(2): 269-275. [ Links ]
18 Hall T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic Acids Symposium Series. 41: 95-98. [ Links ]
19 Hong S.H., Hegde M., Kim J., Wang J., Jayaram A., Wood T.K. 2012. Synthetic quorum-sensing circuit to control consortial biofilm formation and dispersal in a microfluidic device. Nature Communications. 3: 613. doi:10.1038/ncomms1616 [ Links ]
20 Jara S., Maduell P., Orduz S. 2006. Diversity of Bacillus thuringiensis strains in the maize and bean phylloplane and their respective soils in Colombia. Journal of applied microbiology. 101(1): 117-124. [ Links ]
21 Jones S., Yu B., Bainton N.J., Birdsall M.B., Bycroft W.,Chhabra S.R., Cox A.J.R., Golby P., Reeves P.J., Stephens S., Winson M.K., Salmond G.P.C., Stewart G.S.A.B., Williams P. 1993. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. The EMBO Journal. 12(6): 2477-2482. [ Links ]
22 Kado C.I., Heskett M.G. 1970. Selective media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopathology. 60: 969-976. [ Links ]
23 Kalia C.V. 2013. Quorum sensing inhibitors: An overview. Biotechnology Advances. 31(2): 224-245. [ Links ]
24 Kim M.H., Kang H.O., Kang B.S., Kim K.J., Choi W.C., Oh T.K., Lee C.H., Lee J.K. 2005. Crystallization and preliminary crystallographic analysis of Bacillus thuringiensis AHL-lactonase. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 1750(1): 5-8. [ Links ]
25 Leadbetter J.R., Greenberg E.P. 2000. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. Journal of Bacteriology. 18: 6921-6926. [ Links ]
26 Lee S.J., Park S.Y., Lee J.J., Yum D.Y., Koo B.T., Lee J.K. 2002. Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Applied and Environmental Microbiology. 68(8): 3919-3924. [ Links ]
27 Lin Y.H., Xu J.L., Hu J., Wang L.H., Ong S.L., Leadbetter J.R., Zhang L.H. 2003. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Molecular Microbiology. 47(3): 849-860. [ Links ]
28 Liu H., Coulthurst S.J., Pritchard L., Hedley P.E., Ravensdale M., Humphris S., Burr T., Takle G., Brurberg M.B., Birch P.R., Salmond G.P., Toth I.K. 2008. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathogens. 4(6): e1000093. [ Links ]
29 McClean K.H., Winson M.K., Fish L., Taylor A., Chhabra S.R., Camara M., Daykin M., Lamb J.H., Swift S., Bycroft B.W., Stewart G.S., Williams P. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 143(12): 3703-3711. [ Links ]
30 Park S.Y., Lee S.J., Oh T.K., Oh J.W., Koo B., Yum D.Y., Lee J.K. 2003. AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology. 149(6): 1541-1550. [ Links ]
31 Pérombelon M.C., Kelman A. 1980. Ecology of the Soft Rot Erwinias. Annual Review of Phytopathology. 18(1): 361-387. [ Links ]
32 Pirhonen M., Flego D., Heikinheimo R., Palva E.T. 1993. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. The EMBO Journal. 12(6): 2467-2476. [ Links ]
33 Ravn L., Christensen A.B., Molin S., Givskov M., Gram L. 2001. Methods for detecting acylated homoserine lactones produced by gram negative bacteria and their application in studies of AHL-production kinetics. Journal of Microbiological Methods. 44(3): 239-251. [ Links ]
34 Saitou N., Nei M. 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 4(4): 406-425. [ Links ]
35 Schaad N.W., Jones J.B., Chun W.2001. Laboratory Guide of Identification of Plant Pathogenic Bacteria. APS Press (Ed). St. Paul, Minnesota, USA. [ Links ]
36 Sio C.F., Otten L.G., Cool R.H., Diggle S.P., Braun P.G., Bos R., Daykin M., Camara M., Williams P., Quax W.J. 2006. Quorum quenching by an N-acyl-homoserine lactone acylase from Pseudomonas aeruginosa PAO1. Infection and immunity. 74(3): 1673-82. [ Links ]
37 Smadja B., Latour X., Faure D., Chevalier S. Dessaux Y, Orange N. 2004. Involvement of N-acylhomoserine lactones throughout plant infection by Erwinia carotovora subsp. atroseptica (Pectobacterium atrosepticum). Molecular Plant-Microbe Interactions. 17(11): 1269-1278. [ Links ]
38 Tamura K., Nei M., Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences of the United States of America. 101(30): 11030-11035. [ Links ]
39 Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution. 28(10): 2731-2739. [ Links ]
40 Tempé J., Petit A., Holsters M., Van Montagu M., Schell J. 1977. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: Possible relation to transformation in crown gall. Proceedings of the National Academy of Sciences of the United States of America. 74(7): 2848-2849 [ Links ]
41 Thomas P.W., Stone E.M., Costello A.L., Tierney D.L., Fast W. 2005. The quorum-quenching lactonase from Bacillus thuringiensis is a metalloprotein. Biochemistry. 44(20): 7559-7569. [ Links ]
42 Tinh N.T.N., Yen V.H.N., Dierckens K., Sorgeloos P., Bossier P. 2008. An acyl homoserine lactone-degrading microbial community improves the survival of first-feeding turbot larvae (Schopthalmus maximus L.). Aquaculture. 285(1): 56-62. [ Links ]
43 Uribe D., Martínez W., Cerón J. 2003. Distribution and diversity of cry genes in native strains of Bacillus thuringiensis obtained from different ecosystems from Colombia. Journal of Invertebrate Pathology. 82(2): 119-127. [ Links ]
44 Von Bodman S.B., Bauer W.D., Coplin D.L. 2003. Quorum sensing in plant pathogenic bacteria. Annual Review of Phytopathology. 41(1): 455-482. [ Links ]
45 Wang L.H., Weng L.X., Dong Y.H., Zhang L.H. 2004. Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase). Journal of Biological Chemistry. 279(14): 13645-13651. [ Links ]
46 Williams P. 2007. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology. 153(12): 3923-39238. [ Links ]
47 Williams P., Camara M. 2009. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Current Opinion in Microbiology. 12(2): 182-191. [ Links ]
48 Winson M.K., Swift S., Fish L.J., Throup P., Jorgensen F., Chhabra S.R., Bycroft B.W., Williams P., Stewart G. 1998. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiology Letters. 163(2): 185-192. [ Links ]
49 Zhang H.B., Wang L.H, Zhang L.H. 2007. Detection and Analysis of Quorum-Quenching Enzymes against Acyl Homoserine Lactone Quorum-Sensing Signals. Current Protocols in Microbiology. 5:1C.3.1-1C.3.15. DOI: 10.1002/9780471729259.mc01c03s05 [ Links ]