Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Biotecnología
Print version ISSN 0123-3475
Rev. colomb. biotecnol vol.16 no.2 Bogotá July/Dec. 2014
https://doi.org/10.15446/rev.colomb.biote.v16n2.38017
http://dx.doi.org/10.15446/rev.colomb.biote.v16n2.38017
ARTÍCULO DE INVESTIGACIÓN
Optimization of an in vitro regeneration system for colombian indica rice varieties
Optimización de un sistema in vitro de regeneración para variedades colombianas de arroz indica
Iván Darío Barbosa Cepeda1, Alejandro Chaparro-Giraldo2
1 Biologist, MSc. Departamento de Biología, Universidad Nacional de Colombia. idbarbosac@unal.edu.co.
2 Agronomical Engineer, MSc., Ph.D. Associated professor, Departamento de Biología, Universidad Nacional de Colombia.achaparrog@unal.edu.co.
Recibido: enero 10 de 2014 Aprobado: octubre 17 de 2014
Abstract
In order to optimize an in vitro regeneration system for colombian rice varieties, the amount of plant regulators used for each step of the process was modified, together with other factors such as seed position and time of exposition to plant regulators. Modification of 2,4-D concentration during callus induction and coculture has a negative effect on regeneration potential. However, it is possible to increase the amount of produced calli by modifying the position in which the seed is placed on the culture medium. Moreover, a longer callus induction time decreased the regeneration percentage. During the regeneration step, for FEDEARROZ 2000 variety, a combination of 1,0 mg/l g/l mg/l of naftalenacetic acid + 4,0 mg/l g/l mg/l of kinetin increases the regeneration percentage, without increasing the amount of abnormal shoots and without decreasing their size. For FEDEARROZ 50 variety, the best combination of plant regulators is 0,5 mg/l g/l mg/l of naftalenacetic acid + 2,0 mg/l g/l mg/l of kinetin.
Keywords: Colombian rice, FEDEARROZ 2000 variety, FEDEARROZ 50 variety, plant regulators, somatic embryogenesis.
Resumen
Para optimizar un sistema de regeneración in vitro de variedades colombianas de arroz, se modificaron las concentraciones de los reguladores utilizados a lo largo de cada etapa del proceso de cultivo, junto con otros factores como la posición de siembra de la semilla y el tiempo de exposición a los reguladores. Se encontró que la modificación de la concentración de 2,4-D para las etapas de inducción de callo y cocultivo repercute de forma negativa en la regeneración. Por otra parte, es posible incrementar el porcentaje de formación de callo cambiando la posición de siembra de la semilla. Adicionalmente, un mayor tiempo de formación de callo provocó una disminución del porcentaje de regeneración. En la etapa de regeneración, para la variedad FEDEARROZ 2000 se encontró que una combinación de 1,0 mg/l g/l mg/l de ácido naftalenacético + 4,0 mg/l g/l mg/l de kinetina aumenta el porcentaje de regeneración, sin incrementar la cantidad de brotes anormales y sin disminuir de forma considerable el tamaño de los brotes. Para la variedad FEDEARROZ 50, la mejor combinación de reguladores durante la regeneración es de 0,5 mg/l g/l mg/l de ácido natfalenacético + 2,0 mg/l g/l mg/l de kinetina.
Palabras clave: Arroz colombiano, variedad FEDEARROZ 2000, variedad FEDEARROZ 50, reguladores, embriogénesis somática.
Introduction
Rice is one of the most important crop species in the world (Ignacimuthu & Arockiasamy, 2006; FAO, 2012). It is the main food source for nearly half of human population (Sivakumar et al., 2010) and 23% of global caloric consumption comes from this plant (Carsono, 2007). Biotechnology is a valuable tool to meet the current and future demands of rice production (Tariq et al., 2008, Sivakumar et al., 2010), but it cannot replace conventional methods of plant breeding; however, it can help to obtain new genotypes (Poehlman & Allen, 2005, Sharma et al., 2005, Abdul Rahman et al., 2010).
In Colombia, breeding programs have increased the production levels and developed broadly accepted varieties, because of their agronomical and culinary characteristics (Diago, 2003).
The evelopment of regeneration protocols is essential for agricultural biotechnology, especially for genetic engineering (Pérez Molpe Balch et al., 1999, García- Gonzáles et al., 2010). Several factors are important for a succesful Agrobacterium tumefaciens-mediated transformation. However, the availability of an efficient in vitro culture system is critical (Danilova, 2007; Kathuria et al., 2007). There are still some difficulties related with indica rice transformation. This subspecies is considered recalcitrant because of its poor in vitro response and great genotypical variability, compared to japonica genotypes (Saharan et al., 2004a; Amarasinghe & Yang, 2005). Therefore, it is necessary to adjust the protocols almost every time a new genotype has to be transformed, given that no standard protocol has been developed to date (Hoque et al., 2007; Kathuria et al., 2007; Sivakumar et al., 2010). Several factors influence the regeneration potential of an explant: plant genotype, carbon source, mineral nutrients, additives (e.g. vitamins, aminoacids, antioxidants), temperature, relative humidity and even the culture recipient. However, most of the times the response is determined by the interaction between plant genotype, endogenous and exogenous plant growth regulators (Mroginski & Roca 1993; Pérez Molpe Balch et al.; 1999; Pedroza Manrique, 2008).
Recently, the work of Perafán (2011) selected a regeneration protocol (Saharan et al., 2004a) for colombian rice varieties FEDEARROZ 2000, FEDEARROZ 50, FEDEARROZ 369 and CICA 8. However, this protocol needs adjustments (Barbosa & Chaparro, 2011). For that reason, in this work, the response of explants to the artificial plant growth regulators used in in vitro culture was studied, in order to raise the efficiency of the regeneration protocol. Additionally, other factors such as seed position and time of callus induction were evaluated.
Materials and methods
Plant genotypes Mature seeds of indica rice varieties FEDEARROZ 2000 and FEDEARROZ 50 were used. The seeds were kindly provided by the Federación Nacional de Arroceros - FEDEARROZ (National Federation of Rice Growers).
Surface sterilization and culture media
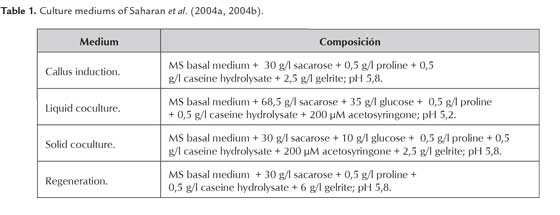
For each experiment, the seeds were manually dehusked and surface-sterilized following a previously established protocol (Barbosa & Chaparro 2011). The initial tissue culture protocol was the one proposed by Saharan et al. (2004a), and selected by Perafán (2011). The coculture step, suggested by Saharan et al. (2004b) was later added, in order to perform Agrobacteriummediated transformation assays. Culture mediums are summarized in Table 1.
Effect of 2,4-D on callus formation
The formation of callus from mature seeds of FEDEARROZ 2000 and FEDEARROZ 50 was evaluated under different 2,4-D concentrations (T1 = 2,5 mg/l; T2 = 3,0 mg/l; T3 = 3,5 mg/l; T4 = 4,0 mg/l; T5 = 4,5 mg/l; T6 = 5,0 mg/l) and compared with a control (T0 = 0 mg/l). 50 seeds in 5 Petri dishes (10 seeds per dish) were placed for each treatment and genotype. Seeds were cultured under dark conditions at 28 °C during 3 weeks. Callus formation percentage was calculated as CFP = (N° of formed calli / N° of seeds cultured) * 100%. Dry weight of each callus per treatment was also determined.
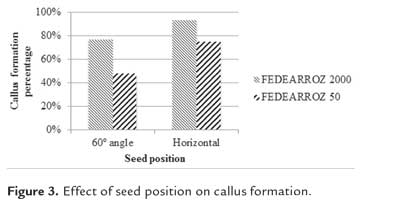
Effect of seed position on callus formation
Mature seeds of both genotypes were manually dehusked and surface sterilized, and then placed on callus induction medium, supplemented with 2,5 mg/l of 2,4-D. Seeds were placed either at 60° (T1) or fully horizontally on the medium. For each treatment and genotype, 10 plates with 10 seeds each were used. Seeds were cultured under dark conditions at 28 °C during 3 weeks. Callus formation percentage was calculated as CFP = (Nº of formed calli/Nº of seeds cultured)* 100%.
Effect of 2,4-D and callus age on regeneration potential
In order to evaluate the effect of 2,4-D and callus age on regeneration potential, mature seeds of both genotypes were placed on callus induction medium, supplemented with different concentrations of 2,4-D (FEDEARROZ 2000: T1 = 3,0 mg/l and T2 = 4,0 mg/l; FEDEARROZ 50: T1 = 3,5 mg/l and T2 = 4,5 mg/l). These concentrations were selected based on previous experimental results and thus were different for each genotype. Seeds were cultured under dark at 28 °C. During 3 weeks, 150 calli were selected weekly and used to simulate coculture conditions according to Saharan et al. (2004b), but without using bacteria. Briefly, shoots and endosperms were removed from the selected calli and they were immersed in liquid coculture medium, supplemented with 2,5 mg/l of 2,4-D, for 30 minutes under constant shaking. Calli were blotted dry on absorbent paper and transfered to solid coculture medium, supplemented with 2,5 mg/l of 2,4-D. Each Petri dish had one Whatman N°1 paper sheet over the medium. Calli were kept under dark conditions at 25°C. After 3 days, they were partially dessicated on Petri dishes containing one Whatman N° 1 paper sheet. Calli were kept under dark conditions at 28 °C for 48 h. Then, the calli were transferred to regeneration medium supplemented with 0,5 mg/l of naphtalene acetic acid (NAA) and 2,0 mg/l of kinetin (KIN). Initially, the calli were transferred to Petri dishes (for each genotype 10 calli per dish, 5 dishes per treatment), and after 3 weeks, they were transferred to 100 mL flasks for 3 additional weeks. During this period, calli were kept under 16 h-photoperiod at 28 °C. For each treatment, shoots were counted (counting only those longer than 5 mm). Regeneration percentage was calculated as RP = (N° of shoots produced/N° of calli cultured)*100%.
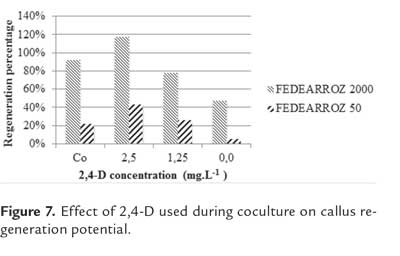
Effect of 2,4-D used during coculture on callus regeneration potential
The effect of 2,4-D concentration during coculture on callus regeneration potential was evaluated by placing seeds on callus induction medium, supplemented with 2,5 mg/l of 2,4-D. Seeds were cultured under dark conditions at 28 °C, during 2 weeks (for FEDEARROZ 2000) or 1 week (for FEDEARROZ 50). Seeds of both genotypes were also cultured during 3 weeks and used as control. After the culture period, the coculture were simulated as previously described, without using bacteria. Three different 2,4-D concentrations were used for liquid and solid coculture media (T1 = 2,5 mg/l ,T2 = 1,25 mg/l and T3 = 0 mg/l ), plus one control for 3 week-old calli (Co = 2,5 mg/l ). For each treatment, 50 calli of each genotype were used. After this, calli were dissecated for 48 h and transferred to regeneration medium supplemented with 0,5 mg/l NAA and 2,0 mg/l KIN, in 100 ml flasks (10 flasks per treatment, 5 calli per flask), under 16 h photoperiod conditions at 28 °C. After 3 weeks, calli were transferred to 200 ml flasks under the same conditions, for 3 additional weeks. For each treatment, shoots were counted (counting only those longer than 5 mm). Regeneration percentage was calculated as RP = (N° of shoots produced/N° of calli cultured)*100%.
Effect of plant growth regulators on callus regeneration potential
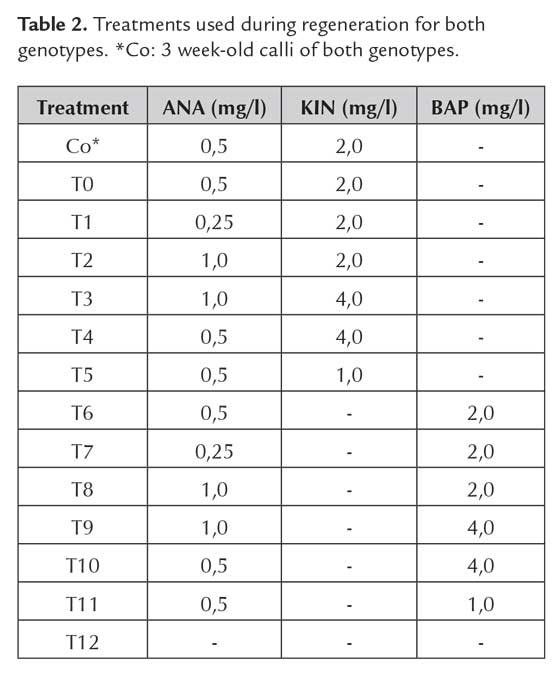
Seeds of both genotypes were placed on callus induction medium, supplemented with 2,5 mg/l of 2,4-D. Seeds were cultured under dark conditions at 28 °C for 2 weeks (FEDEARROZ 2000) and 1 week (FEDEARROZ 50). Seeds of both genotypes were also cultured during 3 weeks and used as control. After the culture period, the coculture was simulated as previously described, without using bacteria. Liquid and solid coculture media were supplemented with 2,5 mg/l of 2,4-D. After this, calli were dissecated for 48 h and transferred to 100 ml flasks with regeneration medium, supplemented with ANA + KIN or ANA + BAP concentrations detailed in table 2.
Calli were cultured under 16 h photoperiod, during 3 weeks (5 calli per flask, 6 flasks per treatment). After this period, calli were transferred to 200 ml flasks for another 3 weeks, under the same conditions. For each treatment, shoots were counted (counting only thoes longer than 5 mm), and regeneration percentage was calculated as RP = (N° of shoots produced/N° of calli cultured) *100%. Shoot dry weight was also determined, and shoots were morphologically classified, according to Pérez Bernal et al. (2007):
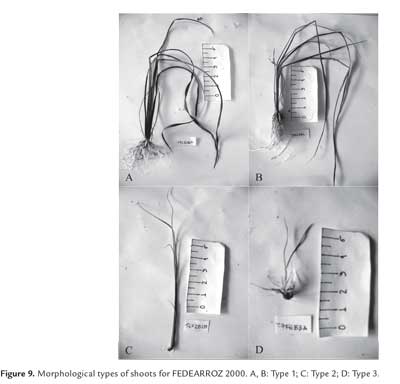
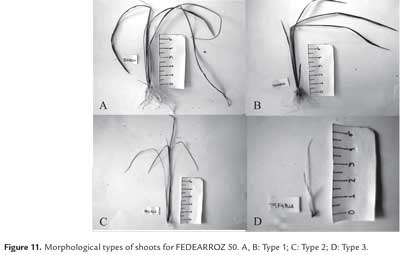
— Type 1: Morphologically normal shoots, with typical bipolar structure, apex and root with proportional length.
— Type 2: Morphologically abnormal shoots, with small or absent root.
— Type 3: Morphologically abnormal shoots, with evident phenotypical alterations, like albinism, very wide or rolled up leaves, or deformed growth axis.
Statistical analysis
For statistical analysis, software Minitab® 16 for Windows™ was used. In all cases, normality tests (Anderson- Darling and Ryan Joiner) were performed. Since data did not adjust to normal distribution and could not be transformed, non-parametrical methods in all analysis were used. For the effect of 2,4-D and callus age on regeneration potential, Friedman test was used. For all the rest of experiments, Kruskal-Wallis test was used.
Results and discussion
Effect of 2,4-D on callus formation
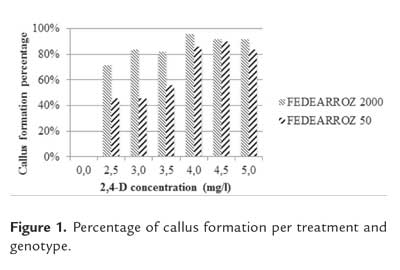
Figure 1 shows the results of callus formation percentage per treatment and varieties. Treatment had a significant effect on the number of produced calli for both genotypes (FEDEARROZ 2000: p<0,001; FEDEARROZ 50: p<0,001). Absence of calli without 2,4-D shows that this regulator is the key factor to stimulate their formation, although some other medium components and environmental aspects could have influence on the morphogenic response (Litz & Jarret, 1993; Mroginski & Roca, 1993; Pérez Molpe Balch et al., 1999; Pedroza Manrique, 2008). FEDEARROZ 2000 had higher callus formation percentages than FEDEARROZ 50. For both genotypes, there were more produced calli with a higher amount of 2,4-D added to the callus induction medium. In contrast, Abayawickrama & Anai (2006) reported that an increase in 2,4-D does not imply a higher amount of formed calli. It must be noticed that there is a slight drop in formed calli from 4,5 mg/l of 2,4-D for FEDEARROZ 2000, and from 5,0 mg/l for FEDEARROZ 50 variety. This drop might be related with the possible toxic and inhibitory effect of high auxin concentrations in the culture medium (Machakova et al., 2008).
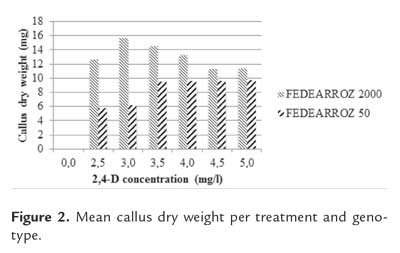
Callus dry weight was used in order to estimate its size. Again, treatment had a significant effect on callus dry weight (FEDEARROZ 2000: p<0,05; FEDEARROZ 50: p<0,001). For all treatments, calli of FEDEARROZ 2000 were larger than calli of FEDEARROZ 50 (figure 2). However, the optimum for dry weight is not the same as for number of calli (figure 1). From 3,0 mg/l , there is a drop in size for FEDEARROZ 2000. FEDEARROZ 50 reaches an optimum dry weight on 3,5 mg/l of 2,4-D and it is kept stable until 5,0 mg/l . The same situation that is observed with FEDEARROZ 2000 can be seen in this case: the optimum treatment for number of calli is not the same for size of calli. This situation has already been reported by Tariq et al. (2008) in mature seeds of four different Basmati varieties.
Effect of seed position on callus formation
Kruskal-Wallis test found significant effects of seed position on callus percentage formation for both genotypes (FEDEARROZ 2000: p = 0,001; FEDEARROZ 50: p < 0,001). Figure 3 shows an important increase of formed calli. FEDEARROZ 2000 goes from 77% to 94%,while FEDEARROZ 50 goes from 48% to 75%. According to Perafán (2011), seeds were placed at a 60° angle over the culture medium. However, placing the seed horizontally has a positive effect on callus induction. This situation might be related with the function of scutellum in a mature embryo. This tissue, and particularly the epithelial cells, absorbs nutrients and growth regulators from endospermum, which are needed for the normal development of the embryo (Vega et al., 2009). It is posible to consider that this position promotes absorption of 2,4-D and thus increases callus formation response. In fact, embryo position in the medium is critical to produce callus from the scutellum of both immature (Jakubeková et al., 2011; Zhong et al., 2011) and mature embryos in maize (Jia et al., 2008), and it has been already reported that seed position has an impact on rice callus production (Amarasinghe & Yang, 2005).
Effect of 2,4-D and callus age on regeneration potential
In order to establish the effect of 2,4-D and callus age on regeneration potential, two 2,4-D concentrations for each genotype were selected, according to the previous experimental results FEDEARROZ 2000: T1 = 3,0 mg/l , T2 = 4,0 mg/l ; FEDEARROZ 50: T1 = 3,5 mg/l , T2 = 4,5 mg/l ). They were selected because a 2,4-D concentration appropiate to maximize the number of produced calli does not correspond to one appropiate to maximize the size of each callus. By choosing two concentrations instead of one, bothvariables -number and size- were taken into account.
Additionally, a control (Co) with the same 2,4-D concentration used in Saharan et al. (2004a, 2004b) was included. According to Perafán (2011), calli were placed on Petri dishes for 3 weeks when transferred to regeneration medium, however, after this time they only developed green buds and spots, with very few shoots (figure 4). For this reason, calli were transferred to 100 mL flasks and sealed with vinylpel, and left under the same environmental conditions for another 3 weeks.
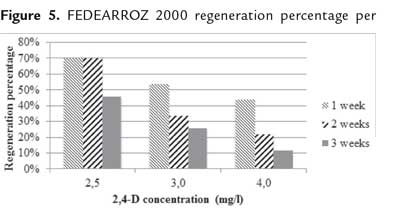
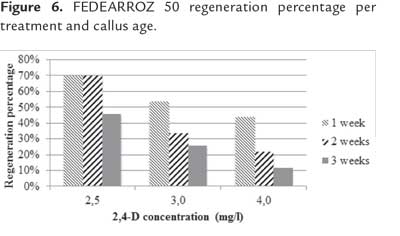
After the additional 3 weeks, produced shoots per each treatment were counted. In both genotypes, Friedman test detected significant differences per 2,4-D concentration and callus age (FEDEARROZ 2000: p = 0,05; FEDEARROZ 50: p = 0,05). Since the p-value in both cases was on the rejection threshold, this result has to be cautiously read. However, figure 5 shows that for FEDEARROZ 2000, the increase of 2,4-D concentration had a negative impact on regeneration potential. While 2,5 mg/l , percentages oscilated between 46% and 70%, 3,0 mg/l percentages oscilated between 26% and 54%, and 4,0 mg/l percentages dropped to 12% - 44%. In addition, callus age also had a negative effect on regeneration potential. Older calli produced less shoots than younger calli. The only exception to this trend occurred with 2,5 mg/l 2,4-D-induced calli, which were able to mantain a 70% regeneration potential until 2 weeks. Figure 6 shows a similar trend for FEDEARROZ 50, regarding 2,4-D concentration and callus age. However, this genotype has a considerably lower regeneration potential than FEDEARROZ 2000, which accounts for the expected high genotypical variation between different indica varieties in response to in vitro culture (Saharan et al., 2004a). Previously, Amarasinghe & Yang (2005) reported that high 2,4-D concentrations and long induction time has a negative impact on callus proliferation capacity.
Although the response of these varieties is different, it is clear that the effect of 2,4-D concentration and callus age is similar for both genotypes. A prolonged incubation time of an explant can cause the loss of its response capacity. It is always best to use young tissues that can respond better to incubation conditions (Litz & Jarret, 1993; Amarasinghe & Yang, 2005). For indirect somatic embryogenesis, one of the main causes of the loss of response capacity is the accumulation of chromosomic aberrations in cells. In fact, several reports indicate that auxins like 2,4-D induce aneuploidies (Singh, 2003). In addition, recent molecular evidence suggests that high concentrations of artificial plant growth regulators in in vitro culture media can trigger the alteration of expression of some miRNAs in calli. This alteration might be the cause of many changes associated to prolonged incubation times, including loss of embryogenic potential (Rodríguez- Enríquez et al., 2011).
In addition, 2,4-D acts not only as an inductor of callus formation, but it also promotes the formation of somatic embryos. Nevertheless, this regulator inhibits the maturation of the embryos, which cannot produce complete plants unless they are transferred to a medium without 2,4-D (Jones & Rost, 1989; Raghavan, 2004; Neumann et al., 2009). High 2,4-D concentrations can promote callus formation, but it can equally cause problems during regeneration since there are residues inside of cells, and these delay or even prevent embryo maturation (Meneses et al., 2005). Additionally, 2,4-D can stimulate ethylen production from explants, which acts as growth inhibitor in in vitro cultures (Machakova et al., 2008). It might be considered, for future experiments, the use of lower 2,4-D concentrations during callus induction in combination with NAA, enough to stimulate the production of calli and, simultaneously, reduce the exposition of somatic embryos to this regulator, which in turn can increase the regeneration potential (Abdul Rahman et al., 2010).
Effect of 2,4-D used during coculture on callus regeneration potential
Kruskal-Wallis test did not find significant differences between treatments in any of the genotypes (FEDEARROZ 2000: p = 0,405; FEDEARROZ 50: p= 0,430). However, figure 7 shows that for both varieties, more shoots were produced in T1 (2,5 mg/l), which is the same amount of 2,4-D used by Saharan et al. (2004b), although with 2 week-old calli for FEDEARROZ 2000 (118%) and 1 week-old calli for FEDEARROZ 50 (44%). Control calli had lower regeneration percentages in both genotypes (FEDEARROZ 2000 = 92%, FEDEARROZ 50 = 22%).
Against what was expected, a reduction of 2,4-D concentration during coculture has a negative impact on callus regeneration potential. For FEDEARROZ 2000, percentages dropped to 78% with 1,25 mg/l and to 48% with 0 mg/l, while for FEDEARROZ 50 they dropped to 26% and 6%, respectively. Visually, calli oxidation levels were higher when the amount of 2,4- D were diminished, therefore it might be considered that this regulator has a protective effect on explants. Generally, the use of 2,4-D has been associated with an increase of oxidation in in vitro conditions, because it stimulates the production of phenolic compounds from the explant (Azofeifa, 2009). However, it has been reported that elimination of 2,4-D in an induction medium might also be associated with oxidation, cell death and extracelular acidification in in vitro culture of Norway spruce (Bozhkov et al., 2002, cited in Machakova et al., 2008).
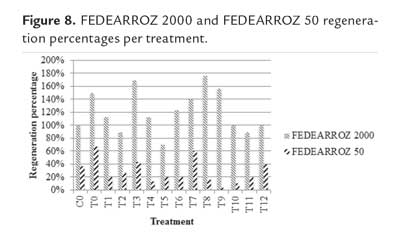
Effect of plant growth regulators on callus regeneration potential
Figure 8 shows the regeneration percentages per treatment and genotype. In both cases, Kruskal-Wallis test did not find significant differences (FEDEARROZ 2000: p = 0,670; FEDEARROZ 50: p = 0,680). However, the graphic shows differences in regeneration values for treatments. First, absence of regulators did not prevent shoot production in both genotypes (T12: FEDEARROZ 2000 = 100%; FEDEARROZ 50 = 40%). Regeneration percentages were similar to those obtained with 3 week-old calli and with the same concentration of ANA and KIN used by Saharan et al. (2004a) (Co: FEDEARROZ 2000 = 100%; FEDEARROZ 50 = 36,7%). It is not always necessary to add regulators to the medium, in order to obtain a morphogenic response (Mroginski & Roca, 1993), and similar results have been reported previously (Sivakumar et al., 2010). Withdrawal of 2,4-D from the medium is, at least initially, the main condition to allow maturation of embryos produced during callus induction (Jones & Rost, 1989; Litz & Jarret, 1993; Neumann et al., 2009). On the other hand, the genotype response to treatments was not equal. Different types of explants and genotypes require different types of cytokinins to have an optimal morphogenic response (Van Staden et al., 2008). For this reason, it is to be expected that BAP and KIN have different effects on calli. BAP induced the highest regeneration percentages for FEDEARROZ 2000 (figure 8, treatments 6-11), which were always higher than 100%, except in T11, where it was 90%. T8 had the highest regeneration percentage, with 176,7%. Regarding KIN treatments, percentages oscilated between 70% and 170%, being T3 the highest (figure 8, treatments 0-5). T2 and T5 had regeneration percentages lower than 100%. Similar results have been reported when comparing treatments of NAA + BAP against NAA + KIN (Pons et al., 2000; Jubair et al., 2008). FEDEARROZ 50 instead, had the highest regeneration percentages in KIN treatments, which oscillated between 13,3% and 66% (figure 8, treatments 0-5). BAP treatments had regeneration percentages between 3,3% and 60% (figure 8, treatments 6-11).
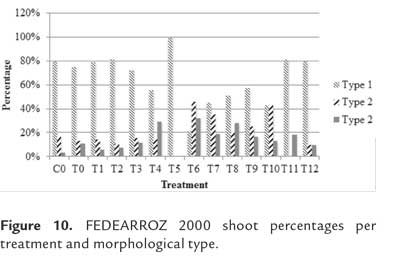
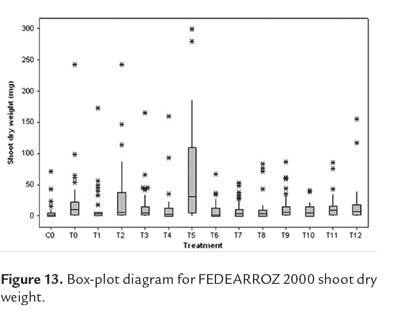
As expected, not all shoots were morphologically equal. Figure 9 shows different types of shoots for FEDEARROZ 2000. Figure 10 shows that FEDEARROZ 2000 produced shoots with some kind of malformation, and that the amount of abnormal shoots varied according to treatment. Only T5 had a 100% normal shoots. Nevertheless, it can be seen that KIN treatments had a higher proportion of normal shoots than BAP treatments. Note that the only BAP treatment with a proportion of normal shoots higher than 80% is T11. Given that 1,0 mg/l were used, it can be assumed that high amounts of BAP causes the formation of abnormal shoots. T8 was the treatment with more shoots produced (176,7%, see figure 8), but only half of them were morphologically normal, and 28,3% were type 3 (figure 10). In comparison, T3 had a regeneration percentage of 170%, and only 11,8% of shoots were type 3. FEDEARROZ 50 had a bigger contrast between proportions obtained for different types of shoots. Figure 11 shows different morphological types of shoots for FEDEARROZ 50. KIN treatments (0-5) produced between 75% and 100% of Type 1 shoots, while BAP treatments produced mostly abnormal shoots (figure 12). Only T11 had a 100% of normal shoots, but this treatment presented only a 20% regeneration percentage. Regarding the rest of BAP treatments, only T7, T8 and 10 produced normal shoots, although merely between 20% and 38,9%. T6, T8 and T9 did not produce any Type 1 shoot. The high proportion of abnormal shoots obtained with BAP, shows that this regulator has an even more negative impact on FEDEARROZ 50 regeneration than on FEDEARROZ 2000. In addition, given that the regeneration percentages were lower than those of KIN reatments, the use of BAP on FEDEARROZ 50 calli can be completely discarded. This type of morphological abnormalities -particularly the absence of roots- have already been reported for indica genotypes, when using BAP as cytokinin (Pipatpanukul et al., 2004; Panjaitan et al., 2009), although normal plants can also be obtained with this regulator (Sivakumar et al., 2010). Shoot dry weight was used, as in the case of calli, in order to estimate size. Kruskal-Wallis test found significant differences between treatments for both genotypes (FEDEARROZ 2000: p = 0,032; FEDEARROZ 50: p = 0,001). Figure 13 shows a high dispersion in shoot dry weight data for FEDEARROZ 2000. However, given the size of boxes and position of medians, it can be seen that, in most of the cases, shoots were small. KIN treatments (T0-T5) produced bigger shoots than BAP treatments, which can be related to the development of a better radicular system, and the consequent formation of normal plants. T5 had the biggest shoots, which might be related to the fact that it also had the highest proportion of normal shoots. Still, this is counteracted by the low regeneration percentage (see figure 8). On the other hand, T3, where the highest regeneration percentage was obtained, had smaller shoots than T5 or even T0. Despite shoot size, the high production of shoots with this treatment constitutesan advantage, compared with the ANA/KIN proportion used by Saharan et al. (2004a). In contrast, boxes and extreme values of BAP treatments are down-displaced, which indicates a smaller dry weight and smaller size. This confirms the negative effect of BAP on callus regeneration.
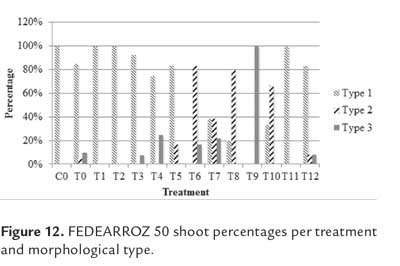
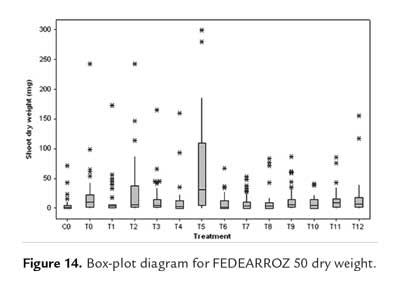
Shoot dry weight data for FEDEARROZ 50 did not have a dispersion as big as for FEDEARROZ 2000 (figure 14). However, again a down-displacement for BAP treatments (T6-T11) can be seen. This result, together with low regeneration percentages (figure 8) and high abnormal shoot production (figure 11), drives to the conclusion that BAP has a negative impact on FEDEARROZ 50 callus regeneration, and should not be used.
Conclusions
This study showed that an increase of 2,4-D during callus induction can increase callus size and callus formation, but it can also reduce the regeneration potential. In this case, no more than 2,5 mg/l during callus induction should be used. The production of calli can be improved by placing the seeds horizontally on the culture medium. In addition, a reduction of callus time induction has a beneficial impact on callus regeneration potential. For FEDEARROZ 2000, calli of up to 2 weeks can be used, whereas for FEDEARROZ 50 calli should not be older than 1 week. On the other hand, a reduction of 2,4-D concentration during co-culture has a negative impact on regeneration potential of both genotypes, for which the same amount suggested by Saharan et al. (2004b) should be used. Finally, for FEDEARROZ 2000, 1,0 mg/l ANA + 4,0 mg/l KIN in regeneration medium increased shoot production, in comparison with regulator treatment used by Saharan et al. (2004a) and Perafán (2011). This was achieved without raising the production of abnormal shoots or reducing the shoot size significantly. For FEDEARROZ 50, the best AUX/CIT combination was the already reported 0,5 mg/l ANA + 2,0 mg/l KIN.
Acknowledgments
First author wants to acknowledge the "Programa de Jóvenes Investigadores e Innovadores Virginia Gutiérrez de Piñeres", of the "Departamento Administrativo de Ciencia, Tecnología e Innovación - Colciencias", for the partial funding of his Master's studies. This work was partially funded by the "Federación Nacional de Arroceros - Fondo Nacional del Arroz (FEDEARROZ - FNA).
Bibliography
1. Abayawickrama, A.; & Toyoaki, A. 2006. Comparison of Regeneration Efficiency of Different Genotypes of Indica Rice Cultivars. Bulletin of Faculty of Agriculture, Saga University. 92: 17-23. [ Links ]
2. Abdul Rahman, Z.; Roowi, S.; Zaliha, W.; Subramaniam, S. 2010. Regeneration of Malaysian Indica Rice (Oryza sativa) Variety Mr232 via Optimised Somatic Embryogenesis System. Journal of Phytology. 2(3): 30-38. [ Links ]
3. Amarasinghe, A.A.Y; & Yang, Y.S. 2005. Comparative studies on invitro respones of fresh and old calli of rice (Oryza sativa L.). The Journal of Agricultural Science. 1 (2): 1-14. [ Links ]
4. Azofeifa, A. 2009. Problemas de oxidación y oscurecimiento de explantes cultivados in vitro. Agronomía Mesoamericana. 20 (1): 153-175. [ Links ]
5. Barbosa, I. & Chaparro, A. 2011. Concentración mínima inhibitoria de higromicina B en callos embriogénicos de arroz (Oryza sativa L.). Revista Colombiana de Biotecnología. 13 (2): 193-198. [ Links ]
6. Carsono, N. 2007. The Establishment of in vitro Culture and Genetic Transformation of the Wheat Glu-1Dx5 Gene to Rice Plants by Gene Gun Bombardment. Tokyo: United Graduate School of Agricultural Science, Tokyo University of Agriculture and Technology (Doctoral thesis), p 107. [ Links ]
7. Danilova, S.A. 2007. The Technologies for Genetic Transformation of Cereals. Russian Journal of Plant Physiology. 54 (5): 569-581. [ Links ]
8. Diago, M. 2003. Compendio de Resultados de Investigación 2001- 2002. Colombia: FEDEARROZ-Fondo Nacional del Arroz. [ Links ]
9. FAO. 2012. FAO Statistical Yearbook. Roma: Food and Agriculture Organization of the United Nations. p 363. [ Links ]
10. García-Gonzáles, R.; Quiroz, K.; Carrasco, B.; Caligari, P. 2010. Plant tissue culture: Current status, opportunities and challenges. Ciencia e Investigación Agraria. 37 (3): 5-30. [ Links ] [ Links ]
12. Ignacimuthu, S.;. & Arockiasamy, S. 2006. Agrobacterium-mediated transformation of an elite indica rice for insect resistance. Current Science. 90 (6): 829-835. [ Links ]
13. Jakubeková, M.; Pret'ová, A.; Obert, B. 2011. Somatic embryogenesis and plant regeneration from immature embryo induced callus of maize (Zea mays L.). Journal of Microbiology, Biotechnology and Food Sciences. 1(4): 478-487. [ Links ]
14. Jia, X.X.; Zhang, J.W.; Wang, H.N.; Kong, W.P. 2008. Efficient maize (Zea mays L.) regeneration derived from mature embryos in vitro. Maydica. 53(3): 239-248. [ Links ]
15. Jubair, T.A.; Salma, U.; Haque, N.; Akter, F.; Mukti, I.J.; Haque, A.K.; Ali, M.R. 2008. Callus Induction and Regeneration of Local Rice (Oryza sativa L.) Variety Topa. Asian Journal of Plant Sciences. 7 (5): 514-517. [ Links ]
16. Jones, T.J.; & Rost, T.L. 1989. The developmental anatomy and ultrastructure of somatic embryos from rice (Oryza sativa L.) scutellum epithelial cells. Botanical Gazette. 150 (1): 41-49. [ Links ]
17. Kathuria, H.; Giri, J.; Tyagi, H.; Tyagi, A. 2007. Advances in Transgenic Rice Biotechnology. Critical Reviews in Plant Sciences. 26: 65-103. [ Links ]
18. Litz, R.E;. & Jarret, R.L. 1993. Regeneración de plantas en el cultivo de tejidos: embriogénesis somática y organogénesis. En: WM Roca, LA Mroginski, editores. Cultivo de Tejidos en la agricultura. Cali: CIAT, p. 143-172. [ Links ]
19. Machakova, I.; Zazimalova, E.; George, E.F. 2008. Plant Growth Regulators I: Introduction; Auxins, their Analogues and Inhibitors. En: EF George, MA Hall, G-J De Klerk, editores. Plant Propagation by Tissue Culture. 3 ed. Dordrecht: Springer, p. 175-204. [ Links ]
20. Meneses, A.; Flores, D.; Muñoz, M.; Arrieta, G.; Espinoza, A. 2005. Effect of 2,4-D, hydric stress and light on indica rice (Oryza sativa) somatic embryogenesis. Revista de Biologia Tropical. 53(3-4): 361-368. [ Links ]
21. Mroginski, L.A.;. & Roca, W.M. 1993. Establecimiento de cultivos de tejidos vegetales in vitro. En: WM Roca, LA Mroginski, editores. Cultivo de Tejidos en la agricultura, Cali: CIAT, p. 19-40. [ Links ]
22. Neumann, K-H; Kumar, A.; Imani, J. 2009. Plant Cell and Tissue Culture - A Tool in Biotechnology. Berlín: Springer, p 333. [ Links ]
23. Panjaitan, S.B.; Abdullah, S.N.A.; Abdul Aziz, M.; Meon, S.; Omar, O. 2009. Somatic Embryogenesis from Scutellar Embryo of Oryza sativa L. var. MR219. Pertanika Journal of Tropical Agricultural Science. 32 (2): 185-194. [ Links ]
24. Pedroza Manrique, J.A. 2008. Aplicaciones del cultivo de tejidos vegetales en condiciones in vitro. Bogotá: Universidad Distrital Francisco José de Caldas, p 348. [ Links ]
25. Perafán, R. 2011. Determinación de un sistema de regeneración para variedades colombianas de arroz (Oryza sativa ssp. indica). Bogotá: Universidad Nacional de Colombia (Tesis de Maestría), p 81. [ Links ]
26. Pérez Bernal, M.; Delgado Rigo, M.; Hernández Díaz, C.A.; Armas Ramos, R. 2007. Evaluación morfológica de brotes regenerados de callos de arroz (variedad IACuba-28) resistentes a higromicina. Revista Colombiana de Biotecnología. 9 (1): 35-40. [ Links ]
27. Pérez Molpe Balch, E.M.; Ramírez Malagón, R.; Núñez Palenius, H.G.; Ochoa Alejo, N. 1999. Introducción al Cultivo de Tejidos Vegetales. Mexico: Universidad Autónoma de Aguas Calientes, p 179. [ Links ]
28. Pipatpanukul, T.; Bunnag, S.; Theerakulpisut, P.; Kosittrakul, M. 2004. Transformation of indica rice (Oryza sativa L.) cv. RD6 mediated by Agrobacterium tumefaciens. Songklanaklarin Journal of Science and Technology. 26 (1): 1-13. [ Links ]
29. Poehlman, J.M.; & Allen Sleper, D. 2005. Mejoramiento Genético de las Cosechas. 2 ed. México: Limusa Wiley, p 512. [ Links ]
30. Pons, M.J.; Marfa, V; Mele, E.; Massager, J. 2000. Regeneration and genetic transformation of Spanish rice cultivars using mature embryo. Euphytica. 114: 117-122. [ Links ]
31. Raghavan, V. 2004. Role of 2,4-Dichlorophneoxyacetic acid (2,4- D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: cell expansion, cell cycling, and morphogenesis during continuous exposure of embryos to 2,4-D. American Journal of Botany. 91 (11): 1743-1756. [ Links ]
32. Rodríguez-Enríquez, J.; Dickinson, H.G.; Grant-Downton, R.T. 2011. MicroRNA misregulation: an overlooked factor generating somaclonal variation? Trends in Plant Science. 16 (5): 242-248. [ Links ]
33. Saharan, V.; Yadav, R.C.; Yadav, N.R.; Chapagain, B.P. 2004a. High frequency plant regeneration from desiccated calli of indica rice (Oryza sativa L.). African Journal of Biotechnology. 3 (5): 256-259. [ Links ]
34. Saharan, V.; Yadav, R.C.; Yadav, N.R.; Ram, K. 2004b. Studies on improved Agrobacterium-mediated transformation in two indica rice (Oryza sativa L.). African Journal of Biotechnology. 3 (11):572-575. [ Links ]
35. Sharma, K.K.; Bhatnagar-Mathur, P.; Thorpe, T.A. 2005. Genetic transformation technology: status and problems. In Vitro Cellular and Developmental Biology-Plant. 41(2): 102-112. [ Links ]
36. Singh, R.J. 2003. Plant Cytogenetics. 2 ed. USA: CRC Press, p 463. [ Links ]
37. Sivakumar, P.; Law, Y.S.; Ho, C.L.; Harikrishna, J.A. 2010. High Frequency Plant Regeneration from Mature Seed of Elite, Recalcitrant Malaysian Indica Rice (Oryza sativa L.) cv. MR219. Acta Biologica Hungarica. 61 (3): 313-321. [ Links ]
38. Tariq, M.; Ali, G.; Hadi, F.; Ahmad, S.; Ali, N.; Shah, A.A. 2008. Callus induction and in vitro Plant Regeneration of Rice (Oryza sativa L.) Under Various Conditions. Pakistan Journal of Biological Sciences. 11 (2): 255-259. [ Links ]
39. Van Staden, J.; Zazimalova, E.; George, E.F. 2008. Plant Growth Regulators II: Cytokinins, their Analogues and Antagonists. En: EF George, MA Hall, G-J De Klerk, editores. Plant Propagation by Tissue Culture. Volume 1. The Background. 3 ed. Dordrecht: Springer, p. 205-226. [ Links ]
40. Vega, R.; Vásquez, N.; Espinoza, A.M.; Gatica, A.; Valdez-Melara, M. 2009. Histology of somatic embryogenesis in rice (Oryza sativa cv. 5272). Revista de Biologia Tropical. 57 (1): 141-150. [ Links ]
41. Zhong, D.Y.; Zhu, Y.Y.; Liu, Q.; Zhou, T.; Zhao, D.G. 2011. Production of Embryogenic Callus and Plant Regeneration from Elite Guizhou Waxy Maize Inbred Lines. Agricultural Sciences in China. 10(4): 490-498. [ Links ]