Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Biotecnología
Print version ISSN 0123-3475
Rev. colomb. biotecnol vol.16 no.2 Bogotá July/Dec. 2014
https://doi.org/10.15446/rev.colomb.biote.v16n2.38747
http://dx.doi.org/10.15446/rev.colomb.biote.v16n2.38747
ARTÍCULO DE INVESTIGACIÓN
Finding of a novel fungal immunomodulatory protein coding sequence in Ganoderma australe
Hallazgo de una nueva secuencia codificadora para una proteína inmunomoduladora de origen fúngico en Ganoderma australe
Andrea González Muñoz1, Kelly Johana Botero Orozco2, Germán Ariel López Gartner3
1 Biologist, Universidad de Caldas; Master's Fellow in Science-Biology, Universidad Nacional de Colombia, Instituto de Genética, Bogotá. Email: andrea_gmu@hotmail.com
2 Biologist, Universidad de Caldas; Investigator, Centro de Bioinformática y Biología Computacional de Colombia, Manizales. E-mail: kelly.botero@cbbc.org.co
3Professor and Investigator, Faculty of Exact and Natural Sciences, Universidad de Caldas, Manizales. german.lopez@ucaldas.edu.co
Recibido: febrero 18 de 2014 Aprobado: octubre 10 de 2014
Abstract
Among the most common human diseases with immune system compromise are autoimmune diseases, cancer, and the acquired immunodeficiency syndrome (AIDS). Many of these diseases still have no treatment or their therapies have undesirable side effects. This has aroused a great interest in the search for new natural products with therapeutic potential and scientifically proven effects, showing minimal side effects. Formal clinical and pharmacological investigation in various medicinal fungi of the genus Ganoderma (Ganodermataceae) has shown immunomodulatory effects and tumor growth inhibition in mammals, attributable to the presence of immunomodulatory proteins and other secondary metabolites. To date, six fungal immunomodulatory proteins (FIPs) have been reported in Ganoderma. This paper seeks to advance in the discovery of immunomodulatory proteins present in Ganoderma australe, through mycelium transcriptome 454 Roche® pyrosequencing (RNA-seq) and bioinformatics analyses. The results suggest the presence of gene sequences related to an immunomodulatory protein which has been reported in another fungal species Taiwanofungus camphoratus. The candidate gene sequences found in G. australe exhibit high identity values in their amino acid composition and predicted protein secondary structure with the protein reported for Tai. camphoratus. According to present knowledge about the action mechanisms of these proteins, it is possible to suggest that this is a promising molecule for the treatment and prevention of diseases associated with certain immune deficiencies, cancer, and other diseases with compromised immune systems. Future studies are proposed in order to determine its immunomodulatory potential using in vitro and in vivo assays.
Keywords: Ganoderma, fungal immunomodulatory protein, immunomodulation, transcriptome, therapeutic.
Resumen
Enfermedades comunes como las autoinmunes, el cáncer y el síndrome de inmunodeficiencia adquirida aún no tienen tratamiento o sus terapias tienen efectos secundarios indeseables. Ello ha suscitado el interés en la investigación de bioproductos con potencial terapéutico, que no impliquen efectos secundarios. Investigaciones farmacológicas y clínicas en algunos hongos medicinales del género Ganoderma (Ganodermataceae) han comprobado efectos inmunomoduladores e inhibidores de crecimiento tumoral en mamíferos, atribuibles a la presencia de proteínas fúngicas inmunomoduladoras (FIPs) y otros metabolitos secundarios. Este trabajo busca avanzar en el descubrimiento de proteínas inmunomoduladoras presentes en Ganoderma australe, mediante la secuenciación del transcriptoma de micelio por tecnología de pirosecuenciación 454 Roche® (RNA-seq) y análisis bioinformáticos. Los resultados sugieren la presencia de secuencias génicas relacionadas con una proteína inmunomoduladora que se ha reportado en la especie de hongos Taiwanofungus camphoratus. Las secuencias génicas candidatas halladas en G. australe exhiben una altos valores de similitud en sus predicciones de composición aminoacídica y estructura secundaria proteica con la proteína reportada para Tai. camphoratus. Los mecanismos de acción de este tipo de proteínas inmunomoduladoras sugieren que se trata de una molécula con potencial promisorio para el tratamiento y prevención de enfermedades con compromiso del sistema inmunológico y el cáncer. Se proponen nuevos estudios que permitan determinar el potencial inmunomodulador de la proteína hipotética hallada mediante estudios in vivo e in vitro.
Palabras clave: Ganoderma, proteína fúngica inmunomoduladora, inmunomodulación, transcriptoma, terapéutica.
Introduction
The Fungi kingdom is considered one of the great biodiversity resources, carrying out important roles in ecosystems and producing chemically diverse secondary metabolites that contribute to a wide variety of bioactive properties in many fungal species. Therefore, fungi are promising resources for the discovery of novel bioproducts with high potential for diverse biotechnological applications.
Interest in the use of therapeutic fungi has been historically restricted to popular medicine and to the manufacture of dietary supplements with health benefits. Moreover, until a few decades ago, the biological potential of these organisms stood as an unexploited resource in formal scientific investigation (Stamets, 1993). However, scientific interest in fungi, as biological resources with biotechnological and therapeutic applications, has increased considerably in the last few decades.
In particular, Ganoderma P. Karsten (1881) is a genus of ancient medicinal fungi, which has been used in traditional medicine for over 4,000 years (McMeekin, 2004) and is considered a "spiritual herb" or "fungi of immortality" in oriental cultures. Within the genus, the species Ganoderma lucidum has been the object of interest of most pharmacological investigations. It has been proven that its extracts and pure biomolecules inhibit cancer cell growth and promote an in vivo immune response through inducing immunoglobulin and cytokine production (Chang et al., 2009; Lin et al., 2006); in addition, its metabolites can be effective antivirals against the human immunodeficiency virus (HIV) (Gao et al., 2003). Recently, one of these species' bioactive therapeutic proteins has been cloned and efficiently expressed in molecular vectors (Wu et al., 2013). Furthermore, other Ganoderma species have also been shown to be effective in diverse biotechnological applications for medicinal use. Ganoderma applanatum, G. tsugae, G. sinense, and G. microsporum also promote antitumor and immunomodulatory activities (Jeong et al., 2008; Liao et al., 2008; Li et al., 2010a; Lin et al., 2010), and G. australe has been reported to possess antimicrobial functions as well (Albino-Smania et al., 2007). The most recent biotechnological applications in this genus include the development of recombinant fungal immunomodulatory proteins (Bastiaan-Net et al., 2013; Zhang et al., 2013) and nanoparticle mycosynthesis for the directed modulation of antibiotic and antimicrobial (Karwa et al., 2011) and antitumor activities (Li et al., 2010a, 2010b).
Pharmacological and formal clinical studies on Ganoderma have investigated its therapeutic activity with further detail and have scientifically evaluated its capacity for modulating the activation and expression of cells and messengers involved in the immune response. This immunomodulation is mediated in part by proteins of the Fungal Immunomodulatory Protein (FIP) family, among other therapeutic secondary metabolites. The biological function and action mechanisms of these proteins have been reported extensively in diverse studies (Li et al., 2011a; Li et al., 2010a; Lin, 2005), classifying them as possible effective agents for treating and preventing diseases originated by certain immunodeficiencies and other states of immune dysfunction.
In general, mature FIPs have been defined as a family of proteins with a molecular weight of around 13 kDa, with 110 to 114 amino acid residues, and exist as homodimers (Lin et al., 1997), except for FIP-gmi which is a tetramer (Wu et al., 2008). In addition, these proteins exhibit a high structural and functional similarity with the immunoglobulin superfamily (Li et al., 2011a). Since the first FIP was isolated and characterized from G. lucidum (LZ-8) (Tanaka et al., 1989), its biological functions have been intensively explored and its DNA and protein sequences have served as references for the identification of new FIPs in other fungi, through homology hypotheses. To date, a total of nine FIPs have been reported from diverse basidiomycetes: FIPfve, from Flammulina velutipes (Ko et al., 1995); FIPvvo, Volvariella volvacea (Hsu et al., 1997); FIP-tve, Trametes versicolor (Li et al., 2011b); LZ-8, G. lucidum (Murasugi et al., 1991; Kino et al., 1989); FIP-gts, G.tsugae (Lin et al., 1997); FIP-gsi, G. sinense (Zhou et al., 2007, 2009); FIP-gja, G. japonicum (GenBank accession AAX98241); FIP-gmi, G. microsporum (Wu et al., 2007); and FIP-gap, G. applanatum GenBank accession AEP68179).
The use of high-throughput genomic and transcriptome sequencing technologies has generated high volumes of biological information, and the increasing development of bioinformatics tools has built the way to finding and identifying novel genes related to proteins of specific interest, as in the case of therapeutic proteins in fungi (Zhou et al., 2009). The main objective of this work is to identify genetic sequences related to fungal immunomodulatory proteins in G. australe, through transcriptome sequencing (RNA-seq) by next generation sequencing technologies (454 Roche® pyrosequencing) and bioinformatics tools. We report the finding of a candidate coding sequence for an immunomodulatory protein in G. australe, exhibiting high sequence identity and secondary structure similarity to a reported immunomodulatory protein in Taiwanofungus camphoratus. The results motivate further detailed biochemical and functional studies of this predicted hypothetical immunomodulatory protein.
Materials and Methods
Fungal culture
A mycelium sample of Ganoderma australe grown on potato-dextrose agar (PDA) was received as a donation from the Universidad Tecnológica de Chocó, Colombia. For biomass production, the fungus was replicated on PDA and malt-extract agar (MEA). Then, these cultures were used to inoculate a liquid culture medium enriched and optimized for Ganoderma, according to the protocol described by Zhou et al. (2007), in order to obtain a clean and pure sample for RNA extraction from mycelium.
cDNA library preparation
Total RNA extraction and messenger RNA (mRNA) purification, copy DNA (cDNA) library preparation, and 454 GS FLX (Roche®) transcriptome sequencing was performed at the Centro Nacional de Secuenciación Genómica (CNSG), Sede de Investigación Universitaria (SIU), at the Universidad de Antioquia, Medellín, Colombia.
Total RNA extraction and mRNA purification from a mycelium sample of G. australe was done using the RNeasy Plant Mini Kit (Qiagen) and Oligotex mRNA Mini Kit (Qiagen), respectively, according to the manufacturer's instructions. Total RNA and mRNA quantification was carried out on a TBS 380 fluorometer (Turner Biosystems) and quality was determined through capillary electrophoresis on an Agilent 2100 Bioanalyzer (Agilent Technologies).
Once optimal mRNA concentration and quality was determined, cDNA library preparation was done according to the Roche® manufacturer protocol. Library quality was determined through capillary electrophoresis (Agilent 2100 Bioanalyzer, Agilent Technologies) and sequencing was performed on half a plate of the 454 Sequencing System GS FLX platform (Roche®).
De novo transcriptome assembly and bioinformatics analyses
Sequenced reads were assembled using the bioinfomatics algorithms employed by the Newbler 2.6 assembler (Roche®). In order to identify mRNA transcripts related to the expression of immunomodulatory proteins in G. australe mycelium, a local BLAST (tblastn) search was performed against a database of GenBankreported FIPs and other immunomodulatory proteins of other protein families, in order to broaden the search for immunomodulatory proteins in G. australe. Non-FIP family proteins were included if they belonged to fungal species and showed significant alignment e-values and high sequence identities to the reported FIPs.
The transcripts with the highest identity to reported immunomodulatory proteins were selected for further bioinformatics analyses using online programs and servers, such as: Translate, for translating a DNA sequence to its six open reading frames and selecting the most probable coded protein (http://web.expasy.org/translate/); MUSCLE (Edgar, 2004), for multiple alignments between the selected translated protein sequences and reported immunomodulatory proteins; Interpro, to predict protein domain architecture of the candidate translated sequences (http://www.ebi.ac.uk/interpro/); and PSIPRED v3.0, for predicting secondary protein structure (http://bioinf.cs.ucl.ac.uk/psipred/).
Results and discussion
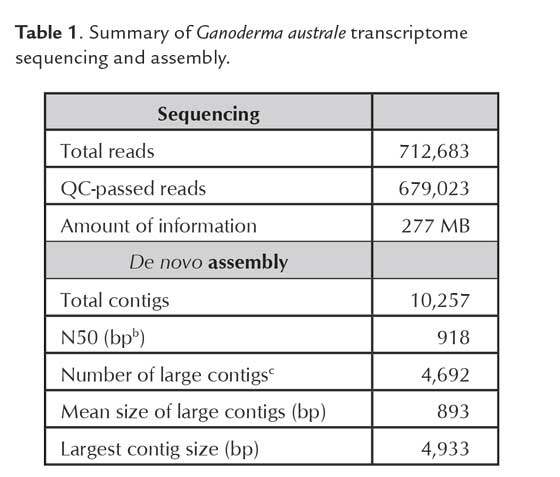
A total of 712,683 raw reads were obtained from the transcriptome sequencing of the mycelium sample of G. australe. Preprocessing and quality control filtered 679,023 reads that were used for de novo assembly. Table 1 summarizes the de novo assembly results.
The search for transcripts (contigs) potentially related to immunomodulatory proteins allowed for the identification of 24 initial candidate sequences showing significant alignments to non-FIP immunomodulatory proteins (data not shown). A further BLAST search against GenBank nonredundant nucleotide and protein databases filtered 12 of these transcripts, showing homology to non-FIP immunomodulatory proteins reported in the basidiomycetes Trametes versicolor (GenBank Accession AGH06133.1) and Taiwanofungus camphoratus (GenBank Accession AAT11911.1), which were not included in our initial protein database, since they showed no significant hit to the FIP family. Although Trametes versicolor has a reported FIP, homology was found with a different non-FIP immunomodulatory protein reported for this species.
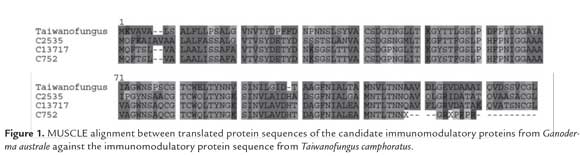
Finally, only the transcripts with the highest identity were selected as definite candidates, which resulted in three candidate contigs: c2535 (e-value=3e-37), c725 (e-value=1e-41), and c13717 (e-value=6e-48), with 69%, 68%, and 66% identities, respectively, to the Tai. camphoratus protein. Subsequent analyses of the Candidates were performed in comparison to this particular Tai. camphoratus protein, due to showing higher identity values than the Tra. versicolor protein. The selected contigs were translated to protein sequences (139 aa, 137 aa, and 137 aa for c2535, c725, and c13717, respectively) and a MUSCLE alignment was performed against the Tai. camphoratus immunomodulatory protein (figure 1). The alignment shows conserved sequence regions between the translated proteins and the reported protein. Furthermore, many mismatch positions included substitutions between amino acid residues with similar biochemical properties.
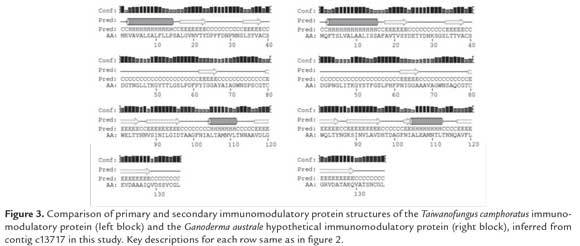
Secondary structure prediction of the hypothetical proteins showed high structural similarity between the three predicted proteins (figure 2). The protein from c13717 was further selected to be compared to the predicted secondary structure of the Tai. camphoratus immunomodulatory protein (figure 3), because it showed the highest prediction confidence level compared to the other two candidates. Both G. australe c13717 and Tai. camphoratus proteins consisted of two α helices and seven b sheets, with the exception of a short additional β sheet in c13717, which was considered non-significant, due to a low prediction confidence level, as represented by the height of the bars above the predicted structure (figure 3). Although secondary structure is not conclusive for determining protein function, it is nevertheless an indicator of a similar folding pattern which could give insight into similar structural motifs between the proteins, as well potential similar function.
Interestingly, the immunomodulatory protein from Tai.camphoratus was isolated by Sheu et al. (2009) from a mycelium extract and reported as a glycoprotein with a molecular weight of 27 kDa and 136 amino acid residues. Its immunomodulatory function is promoted through macrophage activation of a pro-inflammatory response (Sheu et al., 2009). Furthermore, it has been reported that this protein does not promote hemagglutination in humans or mice (Sheu et al., 2009), which makes it promising for therapeutic application.
The immunomodulatory protein from G. australe, inferred from contig c13717 in this study, as well as those reported from Tai. camphoratus and Tra. versicolor, show a predicted protein architecture composed of an 18 amino acid residues signal peptide and a ceratoplatanin conserved domain, which is present in proteins within the cerato-platanin protein family. This family contains phytotoxic proteins of approximately 150 amino acid residues, with four cysteine residues that form two disulfide bridges contributing to protein structure (Pazzagli et al., 1999). Indeed, the immunomodulatory protein predicted from G. australe is not, in a strict sense, an immunomodulatory protein belonging to the FIP family, like other proteins previously reported for Ganoderma. To this respect, strictly speaking FIPs have a molecular architecture composed of immunoglobulin-like beta-sandwich folds, characteristic of proteins within the immunomodulatory fungal family (FIP-fve), which is not the case with the G. australe protein reported in this study. Furthermore, the hypothesized G. australe protein exhibits substantial identity and structural similarity with the Tai. camphoratus protein, and both contain methionine, cysteine, and histidine residues that FIP-fve and LZ- 8-like prototypes do not contain. This evidence supports that the G. australe hypothetical protein reported and modeled in this work is not properly an immunomodulatory protein belonging to the FIP family as traditionally defined, like those reported in other Ganoderma species and other mushrooms, but rather a novel type of immunomodulatory protein with potentially similar functionalities based on its structure.
Finally, the observed homology of the hypothetical protein from G. australe with the immunomodulatory protein from Tai. camphoratus is an interesting finding from a taxonomic perspective, since Tai. camphoratus has shared a taxonomic relation with the genus Ganoderma in the past. G. australe was initially described as Ganoderma comphoratum Zang & Su (1990), yet, due to morphological aspects of the fruiting body, it was reclassified under the genus Taiwanofungus (Polyporaceae) in 1994, with Taiwanofungus camphoratus as the only species in this genus, endemic to Taiwan (Wu et al., 2004).
Further analyses should include the genomic sequencing of G. australe and the in vitro testing of this sequence by means of molecular cloning of the complete gene into an appropriated vector, for example Pichia pastoris, or any other commercial expression vector, in order to further evaluate the therapeutic potential of the gene product through the implementation of protein recombination technology and bioassays approaches using a model organism.
Conclusions
In this study, the presence of a candidate genetic sequence coding for a hypothetical immunomodulatory protein in the mycelium of Ganoderma australe is reported. Moreover, transcriptome sequencing and assembly performed in this study allow an estimation of at least 8,000 to 10,000 genes in G. australe. In order to confirm the presence of the predicted protein and experimentally determine its potential bioactive properties, future prospects include protein isolation and purification, recombinant expression, and possible in vitro and in vivo evaluations of the predicted immunomodulatory function.
Acknowedgements
To the Vicerrectoría de Investigaciones y Posgrados of the Universidad de Caldas for financial support to this project. To Mabel Giselle Torres PhD of the Universidad Tecnológica del Chocó for the donation of the Ganoderma strains and her guidance in fungal culture. To Juan Fernando Alzate PhD and Juan Pablo Isaza of the Centro Nacional de Secuenciación Genómica at the Sede de Investigación Universitaria (SIU) of the Universidad de Antioquia for the sequencing service, transcriptome assembly, and sequence analysis training.
References
1. Albino-Smania, E.F.; Monache, F.D.; Yunes, R.A.; Paulert, R.; Smania-Junior, A. 2007. Antimicrobial activity of methyl australate from Ganoderma australe. Brazilian Journal of Pharmacognosy17(1):14-16. [ Links ]
2. Bastiaan-Net, S.; Chanput, W.; Hertz, A.; Zwittink, R.D.; Mes, J.J.; Wichers, H.J. 2013. Biochemical and functional characterization of recombinant fungal immunomodulatory proteins (rFIPs). International Immunopharmacology. 15(1):167-175. [ Links ]
3. Chang, Y.; Yang, J.S.; Yang, J.L.; Wu, C.; Chang, S.; Lu, K.; Lin, J.; Hsia, T.; Lin, Y.; Ho, C.; Wood, W.G.; Chung, J. 2009. Ganoderma lucidum extracts inhibited leukemia WEHI-3 cells in BALB-c mice and promoted an immune response in vivo. Bioscience, Biotechnology, and Biochemistry. 73(12):2589-2594. [ Links ]
4. Edgar, R.C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 32(5):1792-1797. [ Links ]
5. Gao, Y.; Zhou, S.H.; Huang, M.; Xu, A. 2003. Antibacterial and antiviral value of the genus Ganoderma P. Karst. species (Aphyllophoromycetideae): a review. International Journal of Medicinal Mushrooms. 5(3):235-246. [ Links ]
6. Hsu, H.; Hsu, C.; Lin, R.; Kao, C.; Lin, J. 1997. FIP-vvo, a new fungal immunomodulatory protein isolated from Volvariella volvacea. Biochemistry Journal. 323:557-565. [ Links ]
7. Jeong, Y.; Yang, B.; Jeong, S.; Kim, S.; Song, C. 2008. Ganoderma applanatum: A promising mushroom for antitumor and immunomodulating activity. Phytotherapy Research. 22:614-619. [ Links ]
8. Karwa, A.; Gaikwad, S.; Rai, M.K. 2011.Mycosynthesis of silver nanoparticles using Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.:Fr.) P. and their roles as antimicrobials and antibiotic activity enhancers. International Journal of Medicinal Mushrooms. 13(5):483-91. [ Links ]
9. Kino, K.; Yamashita, A.; Yamaoka, K.; Watanabe, J.; Tanaka, S.; Kerry, K.; Shimizu, K.; Tsunoo, H. 1989. Isolation and characterization of a new immunomodulatory protein, Ling Zhi-8 from Ganoderma lucidum. The Journal of Biological Chemistry. 264(1):472-478. [ Links ]
10. Ko, J.; Hsu, C.; Lin, R.; Kao, C.; Lin, J. 1995. A new fungal immunomodulatory protein, FIP-fve, isolated from the edible mushroom, Flammulina velutipes, and its complete amino acid sequence. European Journal of Biochemistry. 228:244-249. [ Links ]
11. Li, Q.; Wang, X.; Zhou, X. 2011a. Recent status and prospects of the fungal immunomodulatory protein family. Critical Reviews in Biotechnology. 31(4):365-375. [ Links ]
12. Li, F.; Wen, H.; Zhang, Y.; Aa, M.; Liu, X. 2011b. Purification and characterization of a novel immunomodulatory protein from the medicinal mushroom Trametes versicolor. Science China Life Sciences. 54(4):379-385. [ Links ]
13. Li, Q.; Wang, X.; Chen, Y.; Lin, J.; Zhou, X. 2010a. Cytokines expression induced by Ganoderma sinensis fungal immunomodulatory proteins (FIP-gsi) in mouse spleen cells. Applied Biochemistry and Biotechnology. 162(5):1403-1413. [ Links ]
14. Li, N.; Hu, Y.L.; He, C.X.; Hu, C.J.; Zhou, J.; Tang, G.P.; Gao, J.Q. 2010b. Preparation, characterization, and anti-tumour activity of Ganoderma lucidum polysaccharide nanoparticles. Journal of Pharmacy and Pharmacology. 62(1):139-144. [ Links ]
15. Li, S.L.; Hu, Y.P.; Su, J.C.; Song, J.; Guo, Y.W.; Qi, Z.G. 2009. Cloning and expression of LZ-8 gene from Ganoderma lucidum, an immunomodulatory protein. Journal of Hebei Normal University (Natural Science Edition). 33:677–681. [ Links ]
16. Liao, C.; Hsiao, Y.; Lin, C. 2008. Induction of premature senescence in human lung cancer by fungal immunomodulatory protein from Ganoderma tsugae. Food and Chemical Toxicology 46:1851–1859. [ Links ]
17. Lin, C.; Sheu, G.; Lin, Y.; Yeh, C.; Huang, Y.; Lai, Y.; Chang, J.; Ko, J. 2010. A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochemistry. 45:1537-1542. [ Links ]
18. Lin, K.; Kao, Y.; Kuo, H.; Yang, W.; Chou, A.; Lin, H.; Yu, A.L.; Wong, C. 2006. Reishi polysaccharides induce immunoglobulin production through the TLR4/TLR2- mediated induction of transcription factor Blimp-1. The Journal of Biological Chemistry. 281(34):24111–24123. [ Links ]
19. Lin, Z. 2005. Cellular and molecular mechanisms of immune-modulation by Ganoderma lucidum. Journal of Pharmaceutical Science. 99:144–153. [ Links ] [ Links ]
21. McMeekin, D. 2004. The perception of Ganoderma lucidum in Chinese and Western culture. Mycologist. 18(4):165-169. [ Links ]
22. Murasugi, A.; Tanaka, S.; Komiyama, N.; Iwata, N.; Kino, K.; Tsunoo, H.; Sakuma, S. 1991. Molecular cloning of a cDNA and a gene encoding an immunomodulatory protein, Ling Zhi-8, from a fungus, Ganoderma lucidum. The Journal of Biological Chemistry. 266(4):2486-2493. [ Links ]
23. Pazzagli, L.; Cappugi, G.; Manao, G.; Camici, G.; Santini, A.; Scala, A. 1999. Purification, characterization, and amino acid sequence of ceratoplatanin, a new phytotoxic protein from Ceratocystisfimbriata f. sp. platani. Journal of Biological Chemistry. 274: 24959–24964. [ Links ]
24. Sheu, F.; Chien, P.; Hsieh, K.; Chin, K.; Huang, W.; Tsao, C.; Chen, Y.; Cheng, H.; Chang, H. 2009. Purification, Cloning, and Functional Characterization of a Novel Immunomodulatory Protein from Antrodia camphorata (Bitter Mushroom) That Exhibits TLR2-Dependent NF-KB Activation and M1 Polarization within Murine Macrophages. Journal of Agricultural and Food Chemistry57:4130-4141. [ Links ]
25. Tanaka, S.; Ko, K.; Kino, K.; Tsuchiya, K.; Yamashita, A.; Murasugi, A.; Sakuma, S.; Tsunoo, H. 1989. Complete amino acid sequence of an immunomodulatory protein, Ling-Zhi 8 (LZ-8). The Journal of Biological Chemistry. 264(28):16372-16377. [ Links ]
26. Wu, T.Y.; Chen, H.A.; Li, F.Y.; Lin, C.T.; Wu, C.M.; Hsieh, F.C.; Tzen, J.T.; Hsieh, S.K.; Ko, J.L.; Jinn, T.R. 2013. High-level expression, purification and production of the fungal immunomodulatory protein-gts in baculovirus-infected insect larva. Applied Biochemistry & Biotechnology. 169(3):976-89. [ Links ]
27. Wu, C.M.; Wu, T.Y.; Kao, S.S.; Ko, J.L.; Jinn, T.R. 2008. Expression and purification of a recombinant Fip-fve protein from Flammulina velutipes in baculovirus-infected insect cells. Journal ofApplied Microbiology. 104:1354–1362. [ Links ]
28. Wu, M.Y.; Hsu, M.F.; Huang, C.S.; Fu, H.Y.; Huang, C.T.; Yang, C.S. 2007. A 2.0 Å structure of GMI, a member of the fungal immunomodulatory protein family from Ganoderma microsporum. Protein Crystallography. II–132. [ Links ]
29. Wu, S.; Yu, Z.; Dai, Y.; Chen, C.; Su, C.; Chen, L.; Hsu, W.; Hwang, G. 2004. Taiwanofungus, a polypore new genus. Fungal Science. 19(3-4):109-116. [ Links ]
30. Zang, M.; Su, C. 1990. Ganoderma comphoratum. Acta Botanica Yunnanica. 12(4): 395. [ Links ]
31. Zhang, X.; Sun, F.; Liu, Z.; Zhang, S.; Liang, C. 2013. Recombinant Ganoderma lucidum immunomodulatory protein modified with polyethylene glycol. Molecular Medicine Reports. 7(3):975-980. [ Links ]
32. Zhou, X.; Li, Q.; Zhao, J.; Tang, K.; Lin, J.; Yin, Y. 2007. Comparison of Rapid DNA Extraction Methods Applied to PCR Identification of Medicinal Mushroom Ganoderma spp. Preparative Biochemistry & Biotechnology. 37: 369–380. [ Links ]
33. Zhou, X.; Xie, M.; Hong, F.; Li, Q.; Lin, J. 2009. Genomic cloning and characterization of a FIP-gsi gene encoding a fungal immunomodulatory protein from Ganoderma sinense Zhao et al.(Aphyllophoromycetideae). International Journal of Medicinal Mushrooms. 11(1):77-86. [ Links ]