Introduction
In recent years, it has been scientifically demonstrated that plants of the Aloe genus have great nutritional, medicinal and cosmetic value (Pellizzoni et al. 2012). Aloe barbadensis Miller (aloe vera) is the species of greatest importance for the industry because of the great amount of aloe produced by its leaves with the main property aloin, which has multiple medicinal properties (Ramachandra and Srinivasa 2008; Anez and Vásquez 2005).
Conventional propagation of aloe vera is carried out through the separation of suckers or offsets from the base of the adult plants with an estimated rate of re production of 3 to 4 suckers per plant a year (Aggarwal and Barna 2004). Therefore, it is considered to be too slow and insufficient to satisfy the commercial demand (Meyer and Van Staden 1991).
Different methods for aloe vera propagation based on tissue culture techniques have been developed with satisfactory results, which increase the number of plants and accelerate the rate of reproduction (Mukherjee and RoyChowdhury 2008; Supe 2007; Hosseini and Parsa 2007; Matos 2007; Albany et al. 2006; Aggarwal and Barna 2004; Liao et al. 2004; Matos et al. 2000). However, the implementation of this technology through micropropagation on a commercial scale is seriously limited by the high production costs (Savangikar 2004).
The following factors that increase costs in microprop agation of plants are notable: the elevated number of manual operations that entail 40 to 90% labor costs (Pérez et al. 2000; Etienne and Berthouly 2002; Satyahari 2005); estimated electricity expenditure of 60 to 65% of laboratory costs (Ahloowalia and Savangikar 2004; Kodym and Zapata 1999); and the use of gelling agents in the culture media that represent 70 to 90% of the culture medium costs (Prakash et al. 2004; Orellana 1998).
The implementation of propagation methodologies based on the use of liquid culture media that do not re quire the gelling agent definitively reduces production costs (Preil 2005; Aggarwal and Barna 2004). Further more, these media facilitate absorption of nutrients by the tissues; reduce the amount of time required for culture development (Alcaraz-Meléndez et al. 2002); simplify the operations for preparation and release of the media, allowing control and sampling methods to be established in each stage; and they sustain the development of proposals for the automation of in vitro pro cesses (Satyahari 2005; Etienne and Berthouly 2002).
Temporary immersion systems (TIS) were conceived to make the commercialization of micropropagation feasible, because they permit the automation or semi-automation of cultivation systems (Georgiev et al. 2014). Furthermore, primarily subjecting the cultivated plant tissue to alternate cycles of temporary immersion (a few minutes) in the liquid medium followed by drain age and exposure of the tissue to a renewed gaseous environment considerably improves the production and quality of the in vitro cultures (Georgiev et al. 2014; Etienne and Berthouly 2005).
Although the majority of the methods developed for the micropropagation of aloe vera is based on the use of culture media with different gelling agents (Matos 2007; Liao et al. 2004; Matos et al. 2000; Roy and Sakar 1991), promising results have been obtained with the use of liquid culture media using different systems, such as static and agitated systems and those with tem porary immersions, all for the multiplication stage of in vitro micropropagation of aloe vera (Vilchez et al. 2007; Albany et al. 2006; Aggarwal and Barna 2004).
With the aim to dispense with the use of gelling agents in culture media that raise the production costs of the plants and limit commercial micropropagation of aloe vera, the use of the static or temporary immersion liquid medium was compared with the gel medium in the establishment, multiplication and pretransplant stages of in vitro micropropagation of aloe vera.
Materials and Methods
Plant Material
To start the research, 20 to 30 cm-long healthy aloe vera offsets were selected from a plot of adult plants located in the propagator plant beds of the Universidad del Zulia Nursery, Venezuela (10°41'12''N.L. with 71°38'05''W.L. at 36 m.a.s.l.).
In Vitro Establishment Stage
A completely randomized design was used for the experiment with a random arrangement to assess the effect of the static liquid culture medium with a Heller filter-paper bridge and to compare it with the gel medium with 4 g L-1 of agar gel (SIGMA).
The aloe vera offsets selected from the university nursery were firstly washed with tap water and commercial liquid soap, eliminating the remains of substrate and roots. Next, the disinfection procedure described by Albany et al. (2006) was conducted and the 3 cm-long shoots were sectioned until the explant was obtained, comprised of the apical bud of the shoot covered at the base by 3 to 4 leaves with an average height of 1 cm and a diameter of approximately 0.5 cm.
The explants were introduced in test tubes of 150 x 20 mm (Bellco Glass) and covered with polypropylene stops (Bellco Glass INC. Kaputs). These contained 15 ml of the M&S culture medium (Murashige & Skoog, 1962) with pH 5.8, supplemented with 100 mg L-1 of myoinositol, 100 mg L-1 of ascorbic acid, 25 mg L1 of cysteine and 30 g L-1 of sucrose; without the addition of growth regulators.
Ten repetitions of three experimental units per repetition were established for a total of 30 explants in each treatment (liquid medium and gel medium). The experimental unit was comprised of a test tube with an explant and the variables measured were the percentage of explants established at 7, 14 and 21 days of cultivation, the percentage of contaminated explants and the height of the explant at the end of the cultivation period (21 days).
In Vitro Multiplication of Aloe Vera in Recipients for Automated Temporary Immersion (RITA ® )
To assess the effect of the liquid culture medium on in vitro multiplication of aloe vera, commercial recipients called RITA® were selected. It was compared with the gel medium, because this is commonly used in the in vitro multiplication of aloe vera (control).
Two experiments were conducted. The first evaluated the frequency of immersion every 6, 8 and 12 hours with 1 minute of immersion. The second experiment evaluated the immersion time of 1, 2, 3 and 4 minutes with the frequency selected in the first experiment of every 8 hours.
A completely random experimental design was used in each experiment with four repetitions per treatment. For the liquid treatments, 200 ml of culture medium were added to the RITA® and for the control treatment, 50 ml of gel culture medium with 4 g L-1 of agar gel (SIGMA) were added in glass flasks with a capacity of 1 L.
In vitro aloe vera suckers that were 2 to 3 cm long with at least three developed leaves were selected from the fourth cycle of multiplication. Each sucker was adapted in aseptic conditions and an average length of 1 cm was cut from the base toward the distal end of the leaves, and ten explants were planted in each recipient.
The M&S medium (Murashige & Skoog, 1962) was used in the two experiments, supplemented with 100 mg L-1 of myoinositol, 100 mg L-1 of ascorbic acid, 25 mg L1 of cysteine, 1 mg L-1 of Benzylaminopurine (BAP) and 30 g L-1 of sucrose at pH 5.8. The variables measured were the number of suckers per explant and the height of the explant at 30 days of cultivation.
In Vitro Pretransplant of Aloe Vera in Liquid and Gel Media
To compare the effect of the liquid and gel media on in vitro pretransplant, aloe vera suckers with a height of 3 to 6 cm were selected from a seventh in vitro propagation subculture. All lateral buds were removed from these suckers and they were cut across the foliage to reduce the height of the propagules to approximately 3 cm.
The explants were individually placed in test tubes that contained 15 ml of static liquid culture medium with a Heller bridge or gel medium with 4 g L-1 of agar gel (treatments). The M&S culture medium (Murashige & Skoog, 1962) was used with a 50% reduction of the salts, supplemented with 100 mg L-1 of myoinositol, 100 mg L-1 of ascorbic acid, 25 mg L1 of cysteine and 30 g L-1 of sucrose; at pH 5.8.
The percentage of rooted suckers was assessed at 10, 20 and 30 days of cultivation; and at the end of cultivation (30 days) the height of the vitroplant, and the number and the length of the roots were measured. A completely randomized design experiment was used with eighty repetitions per treatment.
General Conditions of in Vitro Growth
The establishment, multiplication and pretransplant stages were developed in an in vitro growth room with an average temperature of 26 °C ± 2 °C with continuous white fluorescent light (40 W) and an intensity of 150 μmol m-2 s-1.
For the statistical analysis, the percentage values of the dependent variables in the establishment and pre-transplant stages were transformed using the equation: n1/2 +½, where n is the percentage value.
The data from all the dependent variables were assessed through the simple analysis of variance and Tukey's range test. All of the statistical analyses were conducted with the Statistix Version 8.0 (2003) computer program for the Microsoft® Windows operating system.
Results and Discussion
In Vitro Establishment in Liquid and Gel Media
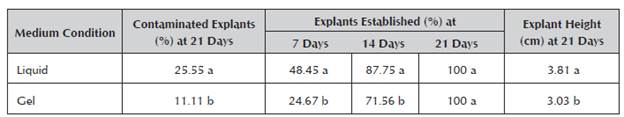
The percentage of contaminated explants, the explant height and the percentage of explants established at 7 and 14 days was affected by the culture medium condition in the in vitro aloe vera establishment stage. However, all the explants that survived managed to establish themselves regardless of the culture medium at 21 days (Table 1).
Table 1 Effect of the static liquid medium with a Heller bridge and gel medium with agar gel on the percentage of contaminated explants and the height of the explants in the in vitro establishment stage of aloe vera.
Values followed by different letters differ statistically (p ≤ 0.05) for Tukey's least significant difference test.
The percentage of contaminated explants observed in the gel medium doubled in the liquid medium. The presence of microorganisms in the liquid media can be easily detected by the colors, textures, uniform or non-uniform turbidity, and films or sediments of the substances that these microorganisms segregate in the medium (Alvarado 1998). Darkening of the medium was observed in the gel media, probably due to the release of phenols that hindered the detection of the contaminants, increasing the risk of losses in stages after establishment, such as multiplication. Therefore, the need to observe the contaminants for their timely elimination is an aspect that must be taken into account even when selecting the gelling agent (Capote et al. 2002). Those that have a translucent appearance similar to the liquid culture medium (phytagel and gel langum) are the most expensive ones.
The percentage of contaminated explants in the in vitro establishment phase of aloe vera in gel culture media has been evaluated, but to demonstrate the influence of sucrose content (Tanabe and Horiuchi 2006) and the effect of the superficial disinfection method used (Albany et al. 2006; Matos et al. 2000). However, the result obtained in this research of 11.11 % of explants contaminated in the gel medium was similar to that reported by Albany et al. (2006) of 9.09 % and less than the one obtained by Matos et al. (2000) with 24 %. In terms of the liquid medium, there are no models to establish appropriate comparisons, and although it was statistically higher than the gel medium in this study, it allowed the contaminated material to be quickly identified and the early exclusion of any source of contamination.
The percentage of explants established at 7 days from cultivation in the liquid medium was higher than in the gel medium (Table 1), a trend that was maintained until 14 days of cultivation. However, all the explants managed to establish themselves by 21 days, regard less of the condition of the culture medium.
These results seem to be related to the ease of the tissues in absorbing nutrients from the liquid media and to manifest growth in little time, while the gel culture media limit absorption of nutrients (Lorenzo et al. 1998), because these are used to form part of the gel matrix and the explants require more time to manifest growth.
Additionally, in the first 7 days of cultivation, the presence of phenolic compounds was observed at the base of the explants in both culture media (liquid and gel). However, there was more blackening on the contact surface with the culture medium in the gel medium due to the lower rate of diffusion. The high content of these phenolic compounds causes oxidation in the culture medium (Matos 2007), hindering the establishment of the in vitro aloe vera, as these phenolic substances are recognized growth inhibitors (Roy and Sakar 1991). These reasons may be related to the decrease in the explants' growth in the gel culture media during the first weeks of cultivation. In contrast, the explants from the liquid medium managed to counteract the negative effects of oxidation, because these compounds are more easily diluted in the liquid medium; affecting the explants' growth to a lesser degree.
To date, the gel medium has only been used for the in vitro establishment of aloe vera with similar results in terms of the explants' growth time, reporting after 15 days (Natali et al. 1990; Matos et al., 2000) and after 4 weeks of cultivation (Liao et al. 2004). The results of this research bring to light the efficiency of liquid culture media for the in vitro establishment stage of aloe vera.
At the end of 21 days of cultivation, a greater height was observed in the explants established in the liquid culture medium compared to the gel medium (Table 1). This response is directly associated with nutrient absorption without greater restrictions in the explants cultivated in the liquid medium.
Similar results to this research were reported by Matos et al. (2000), Natali et al. (1990) and Aggarwal and Barna (2004) with values that oscillated between 2 and 4 cm height of aloe vera explants in the establishment stage, but after 30 days of cultivation.
In Vitro Multiplication of Aloe Vera in Recipients for Automated Temporary Immersion (RITA ® )
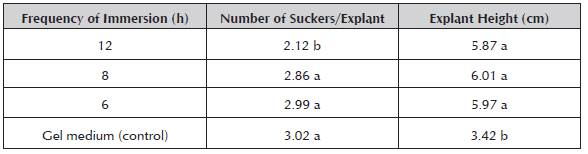
The frequency of immersion during the in vitro multi plication of aloe vera affected the number of suckers from the explants grown in the RITA®, being higher when the frequency was increased to 8 and 6 hours. The number of suckers obtained in the gel medium did not differ. Meanwhile, the explant height was always greater in all the frequencies of the RITA® than in the gel medium (Table 2).
Table 2 Effect of frequency (6, 8 and 12 hours) with 1 minute of immersion in the RITA® on the number of suckers per explant and the explant height in the in vitro multiplication stage of aloe vera.
Values followed by different letters differ statistically (p ≤ 0.05) for Tukey's least significant difference test.
The immersion time in the RITA® only affected the number of suckers per explant, obtaining greater sprouting in a shorter amount of time with an average value similar to that of the control (gel medium). The explant height did not vary between the treatments in the RITA®; but all were higher than the height obtained by the explants grown in the gel medium (Table 3).
Table 3 Effect of immersion time (1, 2, 3 and 4 min) with a frequency of 8 hours in the RITA® on the number of suckers per explant and the explant height in the in vitro multiplication stage of aloe vera.

Values followed by different letters differ statistically (p ≤ 0.05) for Tukey's least significant difference test.
The values obtained for the height of the explant with regards to the number of suckers in both experiments of the RITA® appeared to indicate that the nutrients of the liquid medium are quickly assimilated by the explants, showing greater explant height to the detriment of the formation of suckers. This hypothesis can be confirmed with the behavior of the explants in the gel medium (control), which showed a greater balance between explant growth and the number of suckers grown by them. This was a result of gradual nutrient absorption only by the base of the explant due to the retention exercised by the gel matrix. Meanwhile, in the RITA®, the intermittent contact of the nutritive medium with the explants provides a thin layer of the medium that adheres to the whole surface of the explant by cohesion and it is renewed with each immersion.
In this respect, it has been demonstrated that the conditions of the temporary immersion systems (TIS) and the liquid media in orbital agitation favor greater growth of stems and leaves that cannot be used for the multiplication stage (Albany et al. 2006 and 2005).
In the in vitro multiplication of aloe vera, greater ex plant height has been indicated in liquid culture media in orbital agitation (50 rpm) compared to the gel medium with 3.85 and 2.83 cm, respectively, and a similar multiplication coefficient between both kinds of culture medium with 3.75 suckers per explant (Albany et al. 2006). While Aggarwal and Barna (2004) compared the static liquid culture medium with the gel medium, obtaining 5.1 suckers per explant in the liquid media and 4.8 suckers per explant in the gel media.
It is worth highlighting that the origin of the differences between the number of suckers obtained in this research and those previously indicated is mainly due to the differences between the forms of liquid culture used related to the RITA®, the static liquid medium (Aggarwal and Barna 2004) and medium in orbital agitation (Albany et al. 2006). Furthermore, the composition of the culture media used and the way of measuring the variable strongly supported the differences.
Studies conducted by Vilchez et al. (2007) on the in vi tro multiplication of aloe vera show that a frequency of three times a day for 2 minutes allows a larger number of suckers to be obtained in the RITA® (2.75) and when varying the immersion times (1, 2 and 3 min), differences were not reported in the number of suckers, but a greater explant length was reported (5.01 cm) in the RITA® with 1 minute of immersion three times a day. These results are similar to these shown in this research with frequencies of 8 and 6 hours, which produced the highest numbers of suckers per explant with 2.86 and 2.99, respectively; and when the immersion times were varied (1, 2, 3 and 4 min), a greater number of suckers was observed (2.93) in immersions of 1 minute every 8 hours, with similar height among the explants of the RITA®.
In Vitro Pretransplant of Aloe Vera in Liquid and Gel Media
After 30 days of cultivation in the pretransplant stage, the vitroplant height variables and the number and length of the roots did not show statistical differenc es (p ≤ 0.05) from the effect of the liquid medium in comparison with the gel medium. The percentage of rooted explants showed differences from the effect of the culture medium condition only at 10 and 20 days of cultivation (Table 4).
Table 4 The effect of the static liquid medium and gel medium with agar gel on the percentage of rooted suckers, vitroplant height and the number and length of the roots in the in vitro pretransplant stage of aloe vera.

Values followed by different letters differ statistically (p ≤ 0.05) for Tukey's least significant difference test.
During the first days of cultivation, the difference in the growth of the suckers cultivated in the liquid medium was evident, thus confirming the explants' ease in absorbing nutrients from the liquid medium compared to the gel medium.
At 10 days of cultivation, the percentage of rooted suckers in the liquid medium was double the percentage achieved in the gel medium. However, at 20 days of cultivation, this difference decreased, but a greater percentage of rooted suckers was maintained in the liquid medium. Thirty days later, the suckers placed in gel (Figure 1a) and liquid (Figure 1b) media almost completely rooted, eluding the statistical differences between them.
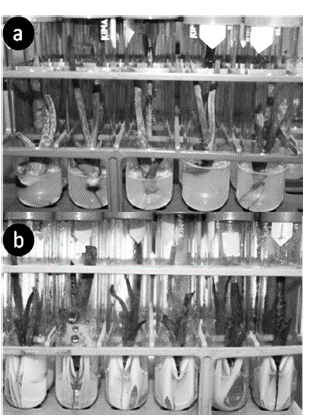
Figure 1 Rooted in vitro aloe vera suckers obtained at thirty days of cultivation. In gel medium systems with 4 g L-1 of agar gel (a). In static liquid medium with Heller bridge (b).
This behavior could be related to the greater resistance provided by the gel medium's matrix to the suckers without roots to absorb the components of the culture medium compared to the liquid medium. It is evident that during the first days of cultivation, the suckers placed in the liquid medium rapidly start to absorb the nutrients, which is why these nutrients of the culture medium are used up at the same speed. For this rea son, as the cultivation time passes, the difference between the percentage of rooted suckers in the liquid medium and in the gel medium starts to decrease.
In this respect, Tanabe and Horiuchi (2006) indicated that the gel concentration influences the aloe vera seedlings' absorption of water and other components of the culture medium. These researchers demon strated that the seedlings' weight-gain percentage increased as the gel concentration decreased, obtaining 74% weight gain with 4 g L-1 of gellan gum.
On the other hand, Orellana (1998) indicates that use of the liquid culture medium permits the diffusion of the vitroplants' toxic waste, primarily the phenolic compounds that are abundant during the initiation of the roots in the pretransplant stage and they decrease the period for the plants to be taken to the transfer-from-culture stage. Furthermore, Gangopadhyay et al. (2002) state that there is a high frequency of root dam age when they are extracted from the gel medium for transplant, and when remainders of gel are left on the roots, it can promote the growth of fungi and bacteria.
In the work on the Aloe genus, a high rooting capacity is observed in the in vitro aloe vera suckers when using different gelling agents with or without the addition of auxins to the culture medium. In this respect, Meyer and Van Staden (1991), reported 100% of suckers of rooted aloe vera with a height between 5 and 6 cm using IBA as a source of auxins and Gelrite as the gelling agent. This was unlike Natali et al. (1990), who used a culture medium without auxins gelled with agar, but who also obtained 100% rooting at 7 and 10 days of cultivation. This was a similar time (approximately 10 days) to that in which Matos et al. (2000) achieved the rooting of aloe vera in a culture medium without hormones and gellan gum as the gelling agent. Mean while, Aggarwal and Barna (2004) obtained 100% rooted suckers of aloe vera in culture media gelled with agar and without auxins after 15 days.
The results stated above indicate that the aloe vera suckers have a high potential to form adventitious roots, confirmed by this research in the liquid culture media, as well as the gel culture media. Having said that, based on the objective of this research to reduce the production costs, it is important to highlight the efficiency achieved in this stage by obtaining 100% of the aloe vera suckers rooted in a culture medium with out a gelling agent and growth regulators, and with a 50% reduction of the M&S salts.
This research constitutes the first scientific contribution to the use of the liquid culture medium in aloe vera for the in vitro establishment stage and the second indication of the use of RITA® in the multiplication stage and the static liquid medium in the pretransplant stage with satisfactory results in all cases.
Conclusions
The micropropagation of Aloe barbadensis Mill. based on a liquid culture medium is simpler and more satisfactory than the gel media with high possibilities of au tomating the in vitro process for commercial purposes.
The static liquid culture with a Heller bridge used in the in vitro establishment and pretransplant stages favors the growth and regeneration of aloe vera suckers, highlighting the reduction of costs of the components of the culture medium, which entails the omission of the gelling agent, the exclusion of growth regulators and the 50% reduction of M&S salts in the pretransplant culture medium in both stages.
In vitro multiplication in the RITA® temporary immersion systems facilitates the absorption of nutrients by the aloe vera explants, translating into greater growth at the same time as the costs of the culture medium are reduced and the opportunity is opened to study other factors to increase the number of suckers in this stage.











 text in
text in 


