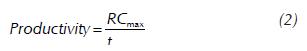Introduction
Milk-clotting enzymes, known as curdling agents or rennet, play an essential role in cheese production. Rennet is obtained as a mixture of chymosin and pepsin from the abomasum lining tissue of calves, where there is a greater proportion of chymosin (88%-94%) depending on the age of the animal. Chymosin is a proteolytic enzyme (EC3.4.23.4) that catalyzes the specific hydrolysis of K-casein in milk in the Phe105-Met106 links. It is classified as a protease of aspartic acid or aspartate protease (EC3.4.23.), which belong to the group of endopeptidase enzymes (Ruiz, 2005). Due to scarcity, high prices, problems associated with animal slaughter and production capacity to satisfy the cheese industry, it has been necessary to search for other alternatives to replace calf rennet (Merheb-Dini, Gomes, Boscolo and Da Silva, 2010). A suitable substitute must demonstrate milk-clotting activity, which consists of a high capacity to attack the 105-106 peptide link of K-casein and low proteolytic activity to minimize the dissolution of clots, thus permitting high yields in cheese-making and products with highly-accepted sensory characteristics.
Research is oriented toward the search for proteases of microbial, animal or plant origin (Merheb-Dini et al. 2010). However, animal and plant proteases have proven to be sources of enzymes of non-specific action in milk clotting, producing cheese with bitter flavors and undesirable textures. Therefore, attention has focused on microbial sources due to their specific action and the advantages of their production, such as short growth periods of microorganisms (Cheng, Bai, and Wang, 2008; Khademi Abachi, Mortazavi, Ehsani, Tabatabaei and Malekzadeh, 2013), unlimited supply and low production costs, as well as it facilitating the design of new enzymatic systems that are not possible to obtain from animal or plant sources. Currently, milk clotting enzymes from fungal sources are used in more than a third of the dairy industry throughout the world (Khademi, Abachi and Malekzadeh, 2013), produced experimentally and industrially by species such as Ther-momucor indicae-seudaticae (Merheb-Dini et al. 2010), Bacillus subtilis natto (Shieh, Phan Thi and Shihb, 2009), Mucor circinelloides (Sathya, Pradeep, Angayarkanni and Palaniswamy, 2009), Rhizomucor nainitalensis (Kh-ademi et al. 2013), Mucor mucedo DSM 809 (Yegin, Fernandez-Lahore, Guvenc and Goksungur, 2010), Rhizomucor pusillus and Rhizomucor miehei (Mahesh-wari, Bharadawaj and Mahalingeshwara, 2000; Jacob,
Jaros and Rohm, 2011; Foda, Moharam, Ramadan and El-Bendary, 2012). The aspartate protease produced by R. miehei is the most commercially used one (Jacob et al. 2011). The following work is presented under this premise with the main objective to evaluate and compare the production capacity of clotting enzymes of five strains of fungi of the Rhizomucor genus.
Materials and Methods
Microorganisms
Strains of Rhizomucor pusillus CVCM-3036 (RP-36), Rhizomucor miehei CVCM-3037 (RM-37) and Rhizo-mucor pusillus CVCM-3039 (RP-39) were used from the Colección Venezolana de Cultivo de Microorganismos (Venezuelan Collection of Microorganism Cultures) and two strains of Rhizomucor spp., identified as BIOMI-12 (Rspp-12) and BIOMI-13 (Rspp-13) from the culture collection of the Microorganism Biotechnology Laboratory of the Universidad de los Andes.
Suspension of Spores
Suspensions of spores were obtained for each strain to be evaluated, using the modified method of Osorio, Gómez, and Sánchez (2008). A volume of 30 ml of sterile distilled water with 25 glass beads measuring 0.5 mm in diameter was added to sporulating mycelia grown in a 250 ml Erlenmeyer flask containing 50 ml of potato dextrose agar medium, incubated for 96 hours at 37 °C, and manually shaken. Once the suspension was recovered, the spore concentration was established by count in a Neubauer chamber.
Production of Milk Clotting Enzymes
In four repetitions, different strains of fungi were grown in a 500 ml Erlenmeyer flask containing 150 ml of the culture medium formulated with casein (4 g/l), glucose (20 g/l), malt extract (4 g/l), bacteriological peptone (4 g/l) and KH2PO4 (2 g/l). They were inoculated with 8x106 spores of each strain and incubated at 37 °C at 150 rpm for 190 hours. Samples of 4 ml were taken every 24 hours, they were centrifuged and filtered in cellulose filter paper of 11 μm porosity and the milk clotting activity and protein and glucose concentration of the supernatant was obtained.
Analysis Techniques
Glucose consumption was quantified using the DNS method for reducing sugars (Miller, 1959). The rate of glucose consumption was estimated by linear regression of the data.
The variation of the protein content during the culture was established by the Bradford method (Bollag and Edelstein, 1991) using bovine albumin as a standard.
The milk clotting activity was measured by the method of Osorio et al. (2008). This consisted of measuring the time that a sample of 5.0 ml of skimmed milk (at 10% in 0.01 M of calcium chloride) takes to clot when adding 0.5 ml of the supernatant of the culture obtained from fermentation. This was established by manually rotating the samples in a bain-marie at a temperature of 37 °C. The reading of the clotting times is taken just when the aspect of the film of milk on the internal wall of the test tube changes from laminar flow to viscous flow, forming small lumps. Enzyme activity was expressed as rennet strength defined as the amount of clotted milk in milliliters per gram or milliliter of supernatant of the culture in 40 minutes at 37 °C. It is calculated through the following ratio:
Where, RS: rennet strength; V: volume of milk (ml); A: amount of culture supernatant (ml); t: clotting time of the sample (s); and 2400: time in which milk normally clots at 37 °C with standard rennet(s).
The proteolytic activity was established using the modified Anson method. Quantifying the soluble peptides that result from the proteolytic activity of the enzyme or of an enzyme extract on bovine casein. Enzyme extract of 1 ml from the culture medium was added to a 4 ml solution of bovine casein at 1%, the reaction mixture was incubated at 37 °C for 20 minutes and the reaction was detained, adding 4 ml of trichloroacetic acid (TCA) at 10%. It was filtered and absorbency was measured at 280 nm. The proteolytic activity unit (U) was defined as the amount of enzyme that catalyzes the conversion of 1 mg of casein per minute. A molar extinction coefficient of 0.81 ml mg-1 was used (Panouille, Nicolai, Benyahia and Durand, 2005).
Production Capacity and Specificity of the Clotting Enzymes
The production capacity was measured through the following ratio:
Where, RSmax: maximum rennet strength achieved; and tRSmax: time in which the maximum rennet strength is achieved.
The specific activity (RSmax/mg of protein) was established as a specificity measurement of crude enzyme extract, and the clotting activity/proteolytic activity index (RSmax /U) by dividing the greatest recorded clotting activity by the proteolytic activity on casein recorded at the same time. The comparison between strains in productivity, clotting activity/proteolytic activity index (RS/U) and the specific activity was conducted by analysis of variance of a single factor for a 5% level of significance and comparison of means through Tukey's test. To do this, the mean and the standard deviation were estimated through three replicas.
Concentration of Crude Enzyme Extracts and Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The crude extracts that presented the greatest rennet strength were saturated with 15% ammonium sulphate, then left to rest for two hours in a water/ice bath and centrifuged at 2900 g for ten minutes. The supernatant obtained was taken to a saturation of 70% and left to rest in water/ice for 12 hours. The precipitate was recovered by centrifugation and dissolved in 1 ml of distilled water and later dialyzed in distilled water.
The concentrated extracts were analyzed in poly-acrylamide gel electrophoresis at 12% in denaturing conditions SDS-PAGE, according to Leammli (1970). In each pool, approximately 20 μg of sample protein and 5 kg of pre-dyed molecular weight marker was added: myosin 194,665 KDa, β-galactosidase 116,531 KDa, bovine serum albumin 97,220 KDa, ovalbumin 50,195 KDa, carbonic anhydrase 37,620 KDa, soybean tryp-sin inhibitor 29,284 KDa, lysozyme 20,010 KDa and aprotinin 7,150 KDa. The molecular weight of each band was calculated using the method of Weber and Osborn (1969).
The enzyme activity of the concentrated extracts was measured in a zymogram according to the modified method of Egito (2007) and Leammli (1970). The resolution gel was prepared with 12% of acrylamide with 0.1% sodium caseinate. The electrophoresis was conducted at 200 volts for 30 minutes, using Mini-Proteam III (Bio-Rad) equipment. To each pool, 20 μg of samples were added. After the electrophoretic migration, the gel was washed two times with Triton X-100 (2% v/v) for 30 minutes. The washed gels were incubated at 37 °C in phosphate buffer (pH 5.4) for 18 hours. After this time, they were dyed in a Coomassie blue solution and later discolored.
Results and Discussion
Production of Clotting Enzymes by the Rhizomucor spp. Strains
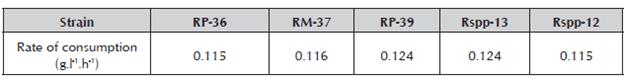
The variation of the glucose concentration of the culture medium during incubation was similar for all the evaluated strains, showing the same trend (Figure 1) and glucose consumption rates (Table 1). Similar behavior was recorded for the Mucor miehei strain in a standard medium using glucose and soluble starch as the main source of carbon (Osorio et al. 2008).
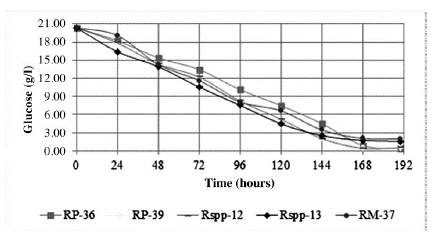
Figure 1 Glucose consumption of the culture medium by the Rhizomucor spp. strains during incubation at 37 °C.
For the first 48 hours, the concentration of proteins in the culture medium decreased from 0.4 mg/ml to approximate values of 0.04 mg/ml for four of the strains (RP-36, RP-37, Rspp-12 and RP-39) (Figure 2), remaining constant until 144 hours when an increase was recorded. In the case of the Rpss-13 strain, a decrease was observed in the concentration of proteins in the first 24 hours (0.11 mg/ml) and an increase at 48 hours (0.148 mg/ml), time in which it decreases to concentrations of 0.05 mg/ml (96 hours), a value that remains constant until the incubation time is completed.
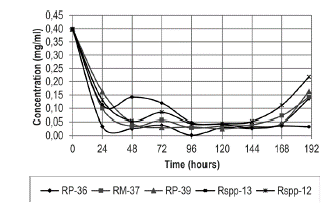
Figure 2 Variation of the protein content of the Rhizomucor strains in the culture medium during incubation at 37 °C .
Increases in the protein concentrations in the culture media (Figure 2) coincided with the maximum clotting activity recorded by the crude extracts of each strain (Figure 3). The greatest value of clotting activity was obtained with the Rspp-13 strain (148.15 RS) at 48 hours, approximately double the value of the activities obtained with the RM-37 and RP-39 strains of 72.73 RS recorded at 190 hours of incubation, while the Rspp-12 and RP-36 strains presented clotting activities of 57.14 RS and 12.50, respectively, in the same conditions. The maximum clotting activity of each strain studied was less than the activity recorded by a strain of Mucor miehei (594.19 RS in 45 hours), cultivated in a standard laboratory culture medium (1.8% soluble starch, 1.8% glucose monohydrate and 0.2% potassium dihydrogen phosphate, 3.1% malt extract, 0.8% bacto peptone and 0.8% casein) (Osorio et al. 2008). In all cases, in the first hours of culture, the casein present in the medium was used as a source of nitrogen for the growth of the strains, while the later increase in protein concentration is attributed to the synthesis and excretion of extracellular enzymes, including those that present milk-clotting activity. The fact that the Rspp-13 strain shows the greatest activity indicates that under the culture conditions used, it has greater capacity for the excretion of proteins, particularly proteases with clotting activity.
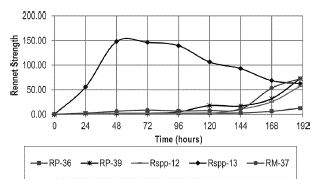
Figure 3 Milk clotting activity recorded by the strains of Rhizomucor spp. during incubation at 37 °C.
In general terms, the trends in the consumption of the main sources of carbon (glucose) and nitrogen (casein), as well as the production of proteases with clotting activity of the strains that were studied, indicate that the glucose consumption rate should not be a factor that influences the excretion of enzymes, not its concentration in the culture medium. At concentrations of 3-4 g/l glucose and 0.05 mg/ml proteins in the medium, a greater increase of excretion is observed in four of the strains. While the Rspp-13 strain in concentrations of 15 g/l and 0.1 mg/l of proteins in the medium produces the greatest excretion of clotting proteins. These results indicate that the levels of glucose (source of carbon) and proteins (source of nitrogen) may be involved in the regulation of protein excretion during growth. Four of the strains are regulated in a similar way. The Rspp-13 strain displays a mechanism that is less suppressed by glucose.
Comparison of Production Capacity
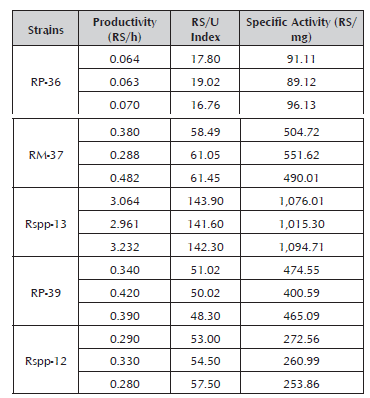
The results displayed in Table 2 indicate that under similar culture conditions, the Rspp-13 strain presents greater productivity, RS/U index and specific activity than the other strains studied. This condition could vary according to the conditions and formulation of the culture medium, and therefore, a wider evaluation of these strains is required.
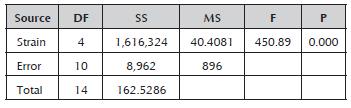
The analysis of variance for the strains' productivity values confirmed the existence of a significant difference between them (Table 3). Comparison of the means established that the Rspp-13 strain with the greatest productivity (3.0857±0.1368 RS/h) and the RP-36 strain with the lowest productivity (0.0657±0.0038 RS/h) presented significant differences between them and with the rest of the evaluated strains, which recorded intermediate values in a range of 0.3000±0.0265 and 0.3833±0.097 RS/h without significant differences between them. The productivity values of the five strains studied were less than the value record by the Mucor miehei strain (13.59 RS/h) (Osorio et al. 2008), probably due to the difference of the media used.
Table 3 Analysis of variance for the productivity of the Rhizomucor strains.
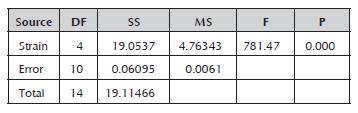
DF: degrees of freedom; SS: sum of squares; MS: mean square; F: test statistic (F-test); and P: statistical significance (p-value).
According to the analysis of variance and the comparison of means for the RS/U indexes, significant differences were found between all of the crude enzyme extracts (Table 4), with the Rspp-13 strain having the greatest index (142.60±1.18) and RP-36 having the lowest index (17.86±1,13). The rest recoded intermediate values of 60.33±1.1; 55.00±2.29 and 49.78±1.38 for the crude enzyme extracts of the RM37, Rspp-12 and RP39 strains, respectively.
Table 4 Analysis of variance for the RS/U index of the crude enzyme extracts of each strain of Rhizomucor.
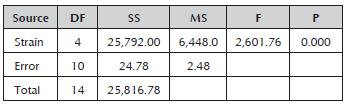
DF: degrees of freedom; SS: sum of squares; MS: mean square; F: test statistic (F-test); and P: statistical significance (p-value).
Like for the previous parameter, the analysis of variance for the specific activity established the existence of a significant difference between the crude enzyme extracts (Table 5). The comparison of means established that the crude enzyme extracts of the Rspp-13 strain of greatest specific activity (1,062.00± 41.5 RS/mg) and the RP-36 strain of least specific activity (92.1±3.6) presented significant differences between them and with the rest of the enzyme extracts, while the RM-37 and RP-39 strains did not present significant differences between them (515.53 ±32.2 and 446.74±40.2, respectively), but the Rspp-12 did, which recorded a specific activity of 262.59±9.4 RS/mg.
Molecular Weight of Enzyme Extracts
The enzyme extract of the partially purified Rspp-13 strain presented two bands of 22.6 and 46.52 KDa (lane 4 - Figure 4a), having a very similar number to those present in the Rspp-12 and RP-36 extracts (lanes 2 and 3 - Figure 4a) of a range from 42 to 50 KDa (upper bands); 22.2 and 20.1, respectively, for the lower bands. These results were different to those reported by Preetha and Boopathy (1997), whose crude and partially purified extracts of Rhizomucor miehei revealed more than two bands in the electrophoresis gel. The RM-37 strain (lane 5 - Figure 4a) presented an additional protein of 194.1 KDa, while the RP-39 extract showed four bands of 23.5; 39.6, 51.2 and 193.8 KDa. Other studies have shown successions of bands in polyacrylamide gel (crude and treated with ammonium sulphate) in extract characterization from cultures of Mucor and Rhizomucor pusillus strains (Nouani, Moulti-Mati, Belbraouet and Bellal, 2011).
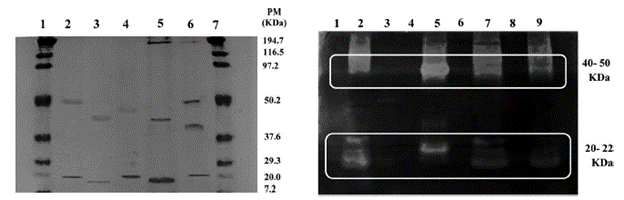
Figure 4 Polyacrylamide gel electrophoresis: (a) SDS-PAGE. Lane: 1 and 7 Molecular weight marker; 2. Rspp-12; 3. RP-36; and 4. Rspp-13; 5. RM-37; and 6. RP-39. (b) Zymogram. Lane: 2. Rspp-12; 3. RM-36; and 5. R-spp-13; 7. RM-37; and 9. RP-39.
All of the profiles of the excreted proteins show the differences between the studied samples, each one demonstrating a characteristic profile. The proteolytic activity present in each extract is associated with the 50 KDa and 22 KDa, proteins corresponding to the Rspp-12, Rspp- 13, RM-37 and RP-39 extracts (lanes 2, 5, 7 and 9 of Figure 4b). This was not the case for the RP-36 extract (lane 3), where the hydrolysis halo can barely be seen. The bands calculated in 194.1 and 193.8 KDa of the RM-37 and RP-39 strains did not present well-defined activity in the zymogram (lanes 7 and 9 of Figure 4b). The intensity of the enzyme activity recorded by the Rspp-13 and RP-36 strains in the zymogram correspond to the production capacity of the clotting enzymes measured in these two strains. The Rspp-13 strain presents the greatest intensity.
Conclusion
All of the strains evaluated in the study demonstrated their capacity to produce milk clotting enzymes except the strains of Rhizomucor pusillus RP-36. The Rhizomu-cor spp. BIOMI-13 strain presented the greatest specific activity, productivity index and RS/U index between the evaluated strains, being the one with the greatest capacity to produce clotting enzymes.











 text in
text in