In memory of Sofía B. Korneva, who devoted part of her work to the development of sugarcane biotechnology in the Bioplant Dept. of the Centro Nacional de Investigaciones Científicas (CNIC) in La Habana, Cuba, as well as in the Center of Biotechnology Research of Ecuador (CIBE, for the Spanish original) belonging to the Escuela Superior Politécnica del Litoral (ESPOL) and in the Research and Development Center of the Unión Nacional de Cañicultores del Ecuador (UNCE).
Introduction
As well as being a sovereign right, the conservation and maintenance of a country's genetic resources is a need that permits the manipulation of the germplasm of a particular crop.
The term germplasm refers to the material that is conserved as seeds, tissue culture or plants established in field collections that groups the intra-specific genetic variability of the genetic materials that can perpetuate the species or population of an organism. There are different ways of conserving the germplasm of a culture. These include the following of note: in situ conservation in banks or gardens, conservation of tissues and plants using in vitro culture media and cryopreservation, a technique that developed with the arrival of in vitro plant tissue culture techniques. Out of these methods, in situ preservation in banks is the most common, extended and easy to conduct (Demey, 2008).
In order to resolve growing food needs, man has managed to increase the production of the different agricultural crops of interest. In this effort, the improvement of plant species for almost all of the crops of economic interest has entailed the repeated use of the best varieties, strains or lines in genetic improvement work. This has resulted in many of the varieties obtained being related to each other, which produces a narrow genetic base. A way to resolve this situation is the use of different genetic forms linked to the species in question, which provide rusticity to said process of genetic improvement.
In sugarcane (Saccharum spp. hybrid), like in the majority of cultivated commercial species, modern hybrids have a narrow genetic base (Costet et al. 2012). One of the objectives of every genetic improvement program is precisely to broaden this genetic base. Therefore, the preservation of the genetic fund (Genofondo) is a priority (Spanish Ministry of Agriculture, 2014).
Establishing a certified biotechnology seed bank is a necessity to provide genetically identified plants free from disease for their later multiplication for commercial, research and genetic improvement purposes. On the contrary, the propagation of untreated plants facilitates the appearance of other foci of infection, which may cause epiphytotic diseases and environmental damage. In the case of Ecuador, "ratoon stunting disease" (RSD) caused by the Leifsonia xili ssp. xili bacterium is one of the main systemic diseases of interest affecting sugarcane.
The objective of this work was to establish the effectiveness of the treatments that allow well-identified initial sugarcane material free from systemic disease to be obtained for its later accelerated propagation, allowing a small, healthy germplasm bank to be established in a relatively short period.
Materials and Methods
The work was conducted in the Research and Development Center that is part of the Unión Nacional de Cañicultores de Ecuador (UNCE), located in Cantón El Triunfo km. 53 Vía Duran Tambo, lower basin of the Guayas Province, Agua Santa Sector, with an average annual temperature of 25 °C, average annual rainfall of 1202.2 mm, relative humidity of 80.1% and an altitude of 60 m.a.s.l., the country's most important sugar producing region.
Identification of Cultivars
A total of eight sugarcane cultivars aged 7-9 months of the most common in Ecuador were used. These were previously identified using morphological characters: Ragnar, CC85-92, RD75-11, CR74-250, BJ70-46, C87-51, SP70-1143 and Mex64-1487. This work confirmed the identification using peroxidase isoenzymes, as described by Arellano et al. 2012). More than three leaves of adult plants of each cultivar used were chosen and they were macerated in liquid nitrogen. Then, a solution that contained 20% sucrose and 0.025% sodium diethyldithiocarbamate (DECA) was added. Electrophoresis in native conditions was conducted using a vertical running system with a polyacrylamide separation gel of 8.5% and a Tris-Glycine running buffer solution of 0.04 M at pH 8.3. The staining method is based on the use of o-dianisidine in presence of the hydrogen peroxide substrate.
First Stage of Sanitation of the Plant Material
The plant material from the sugarcane cultivars used in this work was vigorous and without visible symptoms of the main diseases. The canes with lateral buds from the middle third of the stem were cut using tools disinfected with ethylic alcohol at 70% and carefully washed with running water and detergent without affecting the integrity of the lateral buds. These buds were placed in a bath of hot water at 52 °C for 2 hours. After carrying out the hydrothermal treatment, the buds were then placed in a bath of icy water to detain the treatment and placed in a solution of the fungicide vitavax® 2 g.L-1 for their disinfection. After 20-25 minutes, the buds were incubated at 37 °C for 12-15 days for their germination and covered with moist cotton and gauze. Maintenance was carried out every 48 hours. The germinated buds were planted in small black plastic bags that contained a sterile substrate comprised of soil, rice husks (tamo), sugarcane byproduct and sand in the ratio of 2:1:1:1. Then 2% formol was applied for 15 days, followed by the daily watering of this material. These buds germinated in controlled conditions were treated with the dicopper chloride trihydroxide 2.5 g.L-1 fungicide together with the copper sulfate 0.5 mg.L-1 b bactericide once a week.
Second Stage of Sanitation of Sugarcane Plants and Establishment of the In Vitro Culture
Three to four months after having planted the cleaned buds in the greenhouse, the apical whorls of the plants that had two to three visible nodes were used to obtain the apical meristems. To do this, the bud was disinfected and the apical meristems covered with two to three premature, premature leaves (generally a yellowish color) of a size of up to 4 mm high and 1-2 mm base were extracted and placed in test tubes that contained 10 ml of sterile water heated to 51 °C. Ten minutes later, the hydrothermal treatment of the meri-stems was abruptly interrupted and the tubes with the meristems were placed in cold water at 4 °C and shaken. The aseptic conditions in the establishment medium were maintained in the presence of 0.2 mg.L-1 of 6-benzylaminopurine (BAP), where they were kept for 15 to 20 days at a temperature of 26-28 °C and 6,000 lux of illuminance. Later, the cultures were replanted in the multiplication medium with BAP until their establishment was achieved. This hormone was only added during the multiplication stage.
Third Stage of Sanitation of the Different Sugarcane Cultivars
Once the culture was established, the vitroseedlings were replanted in the propagation medium with the antiobiotic, gentamicin 50 mg.L-1, where they were kept for seven days. Then the plants were replanted in the pretransplant medium with high concentrations (20-30 g.L-1) of sucrose and indole-3-acetic acid (IAA) (1-6 mg.L-1), eliminating those which presented yellow leaves caused by the effect of the antibiotic and those that did not present good characteristics after treatment. The vitroplants that propagated after the second stage of treatment were used to extract the micro-meristems, which had dimensions between 0.5 and 0.8 mm under the stereoscopic microscope. Once the necessary amount of plants was obtained, the pre-transplant stage was carried out, passing to phase I of acclimatization (greenhouse). In all the cases, the synthetic culture media used had the medium described by Murashige and Skoog (1962) as a base with the modifications described by Korneva et al. 1986).
Although healthy plants without evident symptoms of disease were used, the presence of "ratoon stunting disease" (RSD) was assessed in the plants used as starting material for the buds and later in the adult plants obtained from meristems derived from these treatments. The confirmation analysis used for the diagnosis of "ratoon stunting disease" was the presumptive method previously described in the literature, based on the transpiration of the plant tissue and the number of functional and non-functional vascular bundles (Chagas and Tokeshi, 1994). To do this, ten adult stems were taken as a replica of each sapling taken from the cultivar under study and they were cut to ground level, leaving the physiologically active leaves of each variety to assess. For the staining of the vascular bundles, the stems were submerged in a solution of safranin (1.25% p/v) for one hour and then they were removed and left to dry. From the second basal internode, slices of 10 mm in diameter were taken, which were dried at room temperature. Then they were observed in a Boe-co brand stereo microscope for counting the functional and non-functional bundles, which are identified by the presence of red or brown staining, respectively. A Fisher Scientific model Micromaster optic microscope with a built-in camera and an objective of 40x magnification was used for counting the functional and non functional vascular bundles. With these results, the percentage of functional bundles (FB) and non-functional bundles (NFB) was calculated according to Harrison and Davis (1988), using the equation: % FB= FB/TB x 100% and NFB= NFB/TB x 100%, where TB is the total number of bundles assessed.
Additionally, the presence of symptoms corresponding to the systemic leaf scald disease caused by the Xan-thomonas albilineans Ashby bacteria (Dowson) was established.
For the creation of the DNA bank, the genetic material was extracted according to the description by Doyle and Doyle in 1983.
Results and Discussion
The identification of the used cultivars through the patterns of peroxidase isoenzymes is shown in Figure 1. The electrophoretic pattern of each cultivar was unique, as described previously (Arellano et al. 2012). The importance of identifying the cultivars is for preventing the presence of varietal mixes in the fields, a situation that can produce a decrease in the yields or the possibility of unexpected attacks by pathogens in cultivars that are resistant but mixed with another that may be susceptible.
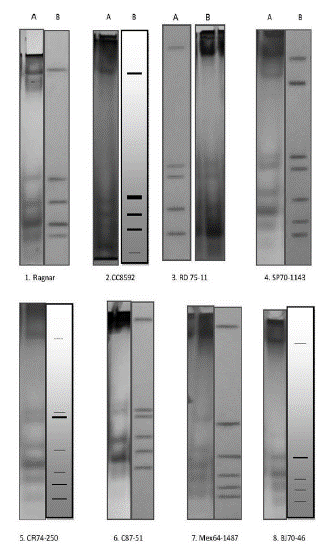
Figure 1 Electrophoresis patterns of peroxidase isoenzymes of the sugarcane cultivars used in this work. A) Electrophoresis pattern of peroxidase isoenzymes; B) Zymogram of the electrophoretic pattern obtained. Cultivar: 1: Ragmar; 2: CC8S-92; 3: RD7S-11; 4: SP70-1143; S: CR74-2S0; 6: C87-S1; 7: Mex64-1487; 8: BJ70-46.
The agro-morphological characters described above are comprised of phenotypical characteristics of easy visual identification or measurement, such as color and height of the stem, shape of the bud and internodes, yield, and susceptibility or resistance to water stress, pests or disease. These characteristics are described as "descriptors" for each culture and are approved by the responsible international organizations.
In the case of biochemical and molecular characteristics, the descriptors will be comprised of the geno-typical expression through the multi-band pattern observed for each individual after their visualization through electrophoresis of proteins or of products of amplification of the DNA molecule. It must be pointed out that in terms of germplasm banks, biochemical and molecular markers have become one of the most useful and most used descriptors in recent times. Therefore, Figure 1 shows the biochemical descriptor used to manage the varieties under study and which was used in this work. It can be appreciated that all the iso-enzymatic patterns distinguish each cultivar studied.
Sanitation and Multiplication of the Sugarcane Varieties for Establishing the Certified Germplasm Bank
Regarding sanitation, during the maintenance of the treated buds, these were closely observed and the fungi that grew on the surface were eliminated using cotton dampened with alcohol. The damp gauze used as support for the buds allowed the system's relative humidity to be maintained. The washing of the lateral buds of the middle third of the stem present in the canes allowed an elevated level of disinfection without affecting the integrity of said buds. The weekly treatments with the dicopper chloride trihydroxide fungicide together with the copper sulfate bactericide also permitted these high rates of disinfection.
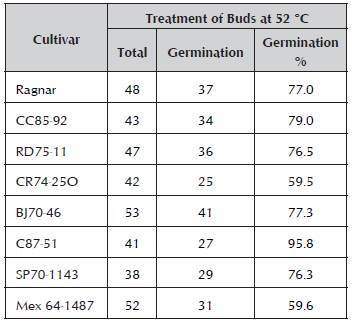
The buds planted in the sterile substrate showed different germination percentages (Table 1). It is observed that the germination percentages go from 59.6% for the Mex64-1487 cultivar to 79% for CC85-92 and 95.8% for C87-51. It is known that increases in the treatment temperature cause a decrease in the germination of buds and survival of plants, but working at lower temperatures does not facilitate the disinfection and sanitation process. Several authors did not achieve germination of the buds in the treatments of 50.5 °C for three hours (Chagas and Tokeshi, 1994). Pérez et al. (1998) reported 30-44% germination using a treatment of 50.5 °C for two hours on propagules of a bud of different sizes and in different cultivars. Chinea and Pérez (1997) obtained 30% germination after applying a similar treatment to individual buds. Pérez and Mauri (1986) only achieved 20% sprouting of buds treated at 50 °C for two hours. In this sense, the results of this work showed much lower mortality values than those previously reported. With respect to sanitation, presence of symptoms of RSD was not observed.
The main objective of sanitation in sugarcane in Ecuador is to eliminate the L. xyli ssp. xyli bacterium, despite a decrease of this disease having been reported in the country (CINCAE, 2011). The diagnosis, based on the presumptive method of staining the vascular bundles previously described by Chagas and Tokeshi (1994) is less effective than other reported methods, but is easy to carry out for a quick diagnosis. Although many of the works cited were from diseased material to confirm sanitation, in our case, the plants used were strong and free from symptoms (Figure 2). It must be noted that the methodology used is not the most modern nor the most sensitive for the detection of bacteria. Therefore, false negatives may be produced, for example, due to the low concentrations of bacteria.
Figure 2 (Healthy) Functional vascular bundles of sugarcane cultivar CC85-92. The red coloring corresponding to the staining used is observed. In the upper-left part, the arrow indicates a damaged vessel.
Several studies on this topic conducted the diagnosis based on the dot-blot analysis and by indirect immuno-fluorescence (IIF). Hernández et al. (1997) detected the presence of RSD, because the thermal treatment was not effective in any case for the elimination of L. xyli.Ramallo and de Ramallo (2001) coincided with these reports, where sanitation of RSD was not achieved with hydrothermal treatment at 50.5 °C for two hours applied to propagules of one bud, although they managed to reduce the bacteria. Similar results were obtained by Pérez et al. (1998), who did not manage to eradicate the pathogen using the same treatment, although they did reduce the bacterial cells. Some of these treatments were not effective for different reasons. Iglesia et al. (2007a) observed dispersion in the data of different determinations of each variety, which could be attributed to the individual response of each plant according to the genotype used. This forces the assessments to cover a higher number of samples than those established. Another cause could be the insufficient number of treatments. Some authors have proposed the need to carry out at least three successive hydrothermal treatments on commercial seed, as well as applying the adequate control measures, to be able to efficiently control RSD. It has been established that a single treatment does not achieve the total inactiva-tion of the organism causing the disease (Chinea and Pérez, 1997; Piñón, 2001). However, it must be taken into account that successive treatments also reduce the germination percentage of the buds.
In this work, although it was not an essential objective, symptoms of the systematic leaf scald disease caused by the Xanthomonas albilineans Ashby (Dowson) bacterium were also not detected. It must be highlighted that cultivar CC85-92, one of the ones with most rapid growth in sugarcane crops in Ecuador, is susceptible to this disease. Therefore, the application of these sanitation techniques combined with the application of accelerated micropropagation through in vitro cultivation of meristems (Arellano et al. 2009) shows a decrease in the incidence of these two systemic bacterial diseases.
Second Stage of Sanitation of Sugarcane Plants and Establishment of the In Vitro Culture
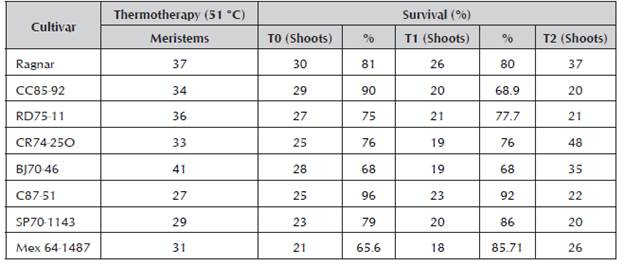
The sanitation of the apical whorls of plants aged three to four months from the buds sanitized in the greenhouse is shown in Table 2. The table shows the number of meristems on which thermotherapy was carried out, out of these, those which survived exiting T0 and their percentage, then those which passed to T1 and survived, and finally the total shoots obtained in T2. It can be appreciated that the survival percentages go from 65.6% for the Mex64-1467 cultivar to 96% for the C87-51 cultivar in the first transfer. This behavior shows the dependence of the cultivar with respect to survival.
In the in vitro tissue culture techniques, the greatest loss of plant material occurs in the first transfers, T0 and T1, due to the establishment of the culture. This decreases from T2, as the plant has completely formed. For this reason, the survival percentage in T0 and T1 was measured.
Despite the controls in the experimental conditions, it was not possible to decrease the losses caused in this stage. The excessive manipulations of the plant material (handling of instruments, excessive heat and inadequate cutting of the meristem) could be some of the causes of the losses. These losses in survival cannot be linked to a reduction of dry mass, which is related to the presence of hyperhydric shoots. According to Kevers et al. (2004), hyperhydricity implies problems of cellular differentiation, because the shoots cannot have a normal morphophysiological development. This phenomenon, which has not been fully explained, has been described as a multicausal disease (Quiala et al. 2012).
The Mex64-1467 cultivar, which during the prolonged treatment at 51 °C resulted in the lowest germination percentage, also showed the lowest survival percentages.
It must be indicated that the use of 6-BAP in the establishment medium facilitated the increase in number of shoots despite the application of prolonged thermal treatment. Use of 6-BAP has been successful in stimulating the proliferation of shoots in different in vitro culture protocols of other cereals (Martinez-Medina et al. 2012; Medina et al. 2012; Syamala and Devi, 2003).
Third Stage of Sanitation of Different Sugarcane Cultivars
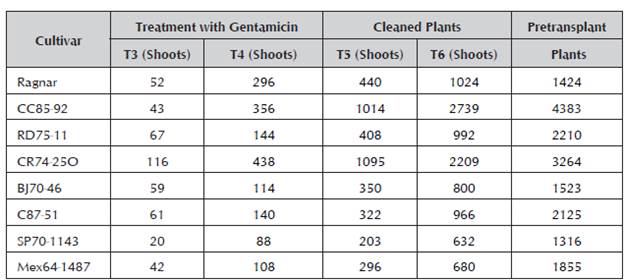
The following step of replanting the plants in the propagation medium with 50 mg.L-1 of the gentamicin antibiotic allowed an increase in the number of shoots, the number of cleaned plants and consequently, the number of plants in the pretransplant phase (Table 3). The inclusion of this wide spectrum antibiotic facilitated the decrease of exogenous contamination. With these work conditions, this step entails the use of antibiotics and the use of micro-meristems as a third treatment. The table shows the increase in the number of plants in the successive transfers, from T3 to T6, which achieved up to thousands of clean and rooted plants.
As previously mentioned, these experiments used material free from diseases or at least without any visual symptoms of them. In these circumstances, it is difficult to refer to sanitation if it uses plants that appear healthy and strong, but the initial idea was to ensure the creation of a germplasm bank ready for immediate use as basic seed.
Meristem apices have been used for the micropropagation of sugarcane. Victoria et al. (1998) managed to eliminate the bacteria causing the stunting by using combined hydrothermal treatments prior to the cultivation of meristems. Similar results were obtained by Peteira et al. (1992), who achieved 96-100% sanitation when combining the cultivation of apices with thermo-therapy (50 °C for 10 minutes). The use of combined systems has achieved the elimination of RSD and also of other similar pathogens of sugarcane, such as the bacteria that cause leaf scald and sugarcane mosaic virus (Matsuoka et al. 1988; Sordi and Tokeshi, 1988; Matos, 2002; McGuire et al. 2009).
Regarding the diagnosis, although the method used was the staining of the vascular bundles (STM) described by Chagas and Tokeshi (1994), it must be pointed out that its presumptive nature facilitates analysis and elevation of the sample volumes because of its relative simplicity, but it may cause confusion with other pathologies. The method has been previously used as an initial alternative to diagnostic work (Iglesia et al. 2007a) and despite the lack of accuracy provided by the non-specific nature of the symptoms of this disease, the simple nature of the method facilitates its implementation and direct execution in the field, as well as the limited need for equipment to count the functional and non-functional bundles. In turn, it provides a scale to interpret the results.
A possibility provided by the germplasm banks is to conserve and move high quality phytosanitary material. The use of thermotherapy together with the disinfection of the work material is an old but very efficient practice to eradicate the pathogens that move systemically across the plant material. In the case of working with in vitro meristems, their small size and speed of propagation extraordinarily reduce the probability of the development of these systemic pathogens; mainly bacteria or viruses. In combination with this, the existence of substances that obstruct the development of pathogens is known, in particular, the presence of guanine, which according to the studies conducted, appears to have antimicrobial activity (Shuravlev, 1979).
The conservation of phytogenetic resources, understood as hereditary material with economic, scientific or social value contained in the species, is of capital importance in the fight against different disasters, such as hunger and global malnutrition, but also the genetic decay of the crops and the loss of genetic diversity. This is where the germplasm and gene banks play an important role. The germplasm banks protect the source of variability required for the plant enhancers for the development of cultivars that allow farmers to overcome the natural limitations with the aim to obtain greater benefits from their activity, as well as protecting the source against genetic erosion (Demey, 2008). The studies on genetic diversity in these gene banks are one of the tools that help to have more effective control over genetic erosion. They allow the variation patterns to be established that determine the incorporation of individuals into genetic improvement programs, whether because of their promising characteristics or susceptibility to biotic or abiotic conditions, facilitating the incorporation of genes and the establishment of the best reproduction strategy. This ensures elevated genetic quality.
An important aspect that must also be indicated as a result of this work is the maintenance of an in vitro germplasm bank with the varieties present in the in situ bank in the field. Although details to complement this are not shown in this work, the gene bank was conserved by maintaining the DNA at temperatures of -20 oC for prolonged periods. This creation of the in vitro banks and the "DNA bank" allows genetic material to be conserved in greatly reduced spaces for long periods of time regardless of the environmental conditions.
Finally, a methodology is proposed to obtain agamic material from sugarcane, where hydrothermal treatment is combined with the cultivation of buds through the micropropagation of meristems in different stages. This methodology proposal is essentially directed at the systemic bacterial disease of sugarcane, RSD (Lei-fsonia xyli ssp. xyli Davis), although it may facilitate the decrease of other systemic diseases of the crop such as LSD (Xanthomonas albilineans Ashby Dowson), which is frequently occurring in an increasing cultivar in Ecuador, CC85-92, as well as sugarcane mosaic virus (SCMV). Regarding the method of confirming sanitation, this constitutes the first approach, as it must be completed with the analysis based on the molecular methods (PCR) for both bacterial diseases. These results are in the phase of being complemented with the molecular method based on the polymerase chain reaction (PCR) with the use of specific primers of the Leifsonia xyli spp. xyli bacterium and with the immu-nochemical method, ELISA. These are currently the most used methods and they posses high efficiency of detection (Iglesia et al. 2007b; Taher-Khani, 2010). The use of strong material that appeared to be free from disease prevented the certainty of whether sanitation was effectively carried out. However, the fact that symptoms did not appear confirmed how clean the material was, even when the presumptive method of staining the non-functional bundles was used.
Regarding micropropagation, the condition of physiological rejuvenation that it introduces and that has been described since the first in vitro propagation studies on meristems is known.
All of these data constitute important information to achieve adequate management of the use of phytogenetic resources of sugarcane.
In addition to the above, this work may constitute a strategy to prevent or decrease the dissemination of these systemic diseases by the delivery of a high quality, phytosanitary and genetically well identified seed to the sugarcane growers like the one obtained by these procedures. Similarly, this seed may constitute an ideal material for genetic exchange between countries.
Conclusions
A methodology is proposed for obtaining a seed of high genetic quality that combines hydrothermal treatment with manipulations of the meristems, which facilitate the sanitation process.
A donor bank was established which serves as a material to plant seedbanks for basic seed, allowing the control of the spread of some systemic diseases such as RSD.











 text in
text in