Introduction
Phosphorus is one of three essential macronutrients and a key component of molecules such as nucleic acids, phospholipids and ATP, as well as forming part of the majority of organisms' physiological processes. However, in plant nutrition, it is an element that limits plant growth because it is available in forms with limited availability for plants (White and Hammond, 2008; Boschetti et al. 2003).
As an agricultural country, Colombia requires new techniques to considerably increase productivity and decrease the intensive use of chemical fertilizers, which supply the amount of nutrients necessary for the plants' growth and development. In the case of phosphorus, deficiencies of up to 98% are reported for plants. Therefore, a large portion of the inorganic phosphates applied as fertilizers are inaccessible for the plant due to the rapid formation of insoluble complexes caused by the different types of soil, Andisol, Inceptisol, Mollisol, Oxisol, Ultisol and Vertisol, characterized by low pHs and a high fixation of P (Galvis, 2001; Sierra, 2002; Rao et al. 2004; Vyas et al. 2007; Oberson et al. 2011; Reed et al. 2011; Botiva, 2012). For this reason, the common practice in the country is to add chemical or organic fertilizers or composts supplemented with phosphate enhancers with the aim to compensate the deficiencies of this element (Burbano and Silva, 2010). Traditional production of chemical phosphatic fertilizers includes treatments with negative effects on the environment as a result of the extraction of phosphates with acids at high temperatures. Parallel to this, processes of eutrophication and erosion are also observed with the application of the chemical fertilizers (Vassilev and Vassileva, 2003; Son et al. 2006; Hamdali et al. 2008a; 2008b; 2008c).
Colombia has 14% of the world's biodiversity and it has the largest number of species per area in the plan et, which suggests that this diversity of flora and fauna could also be represented in Colombian soil at a mi crobiological level (Ghazanfar et al. 2010). The above permits the proposal of the use of this kind of organ ism as a biofertilizer, being appropriate technology for the environment to help reduce the deficiencies in this element in soils for agricultural use (Rodríguez and Fraga, 1999; Vassilev et al. 2006; Vyas et al. 2007; Xiao et al. 2008; Bhattacharyya and Jha, 2012). There fore, this study assessed the in vitro phosphorus solubilization of Streptomyces sp. under three sources of insoluble phosphorus and different initial pH conditions and identified the organic acids involved in the solubilization process presented by these isolations.
Materials and Methods
Identification of Isolations
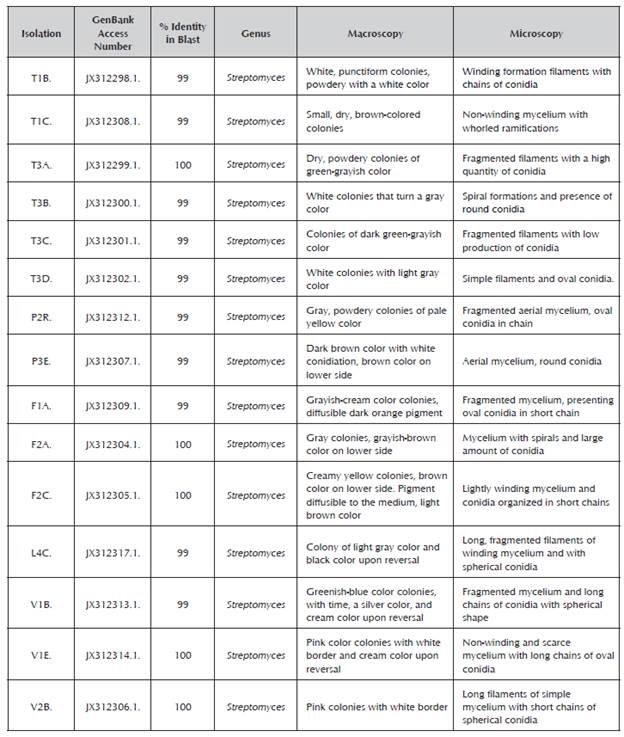
Fifteen isolations of actinobacteria were assessed in this study. The isolations were previously selected from soils in the Eastern Andes of Colombia for their phenotypical characteristics and their phosphate solubilizing capacity according to the description by Salcedo (2014). The isolations were obtained from the rhizo-sphere of native plants purified in oatmeal medium supplemented with nystatin (0.1% v/v). The identification was based on their morphological characteristics and on the sequence of the 16S rDNA gene.
The DNA was obtained according to the procedure described by Kumar et al. (2010) with some modifications. The DNA was extracted from the inoculant of pure homogenized culture biomass in 15 ml of TE 1X (Tris 10 mM; EDTA 1 mM; pH 8). With the addition of lysozyme (20 mg-ml-1), 500 μl of the biomass were lysed and incubated at 37 °C for 30 minutes. Later, 1% SDS (p/v) and Proteinase K (0.1 mg-ml-1) were added and it was incubated again at 55 °C for 30 minutes. After the cellular lysis, the DNA was extracted by the addition of the same volume of phenol-chloroform-isoamyl alcohol (25:24:1), a procedure carried out twice; and later washed with chloroform-isoamyl alcohol (24:1) (v/v). The precipitation was carried out with double the volume of pure ethanol. Finally, the strand of DNA obtained was washed with ethanol at 70% (v/v) and resuspended in 50 μl of distilled molecular grade H2O.
The DNA fragments of the 16S rDNA gene were amplified by PCR from the extraction of DNA according to the modified method of Kumar et al. (2010). Making use of the oligonucleotides fD1 (5'-AGAGTTTGATCCTGGCT-CAG-3') and rP2 (5'-ACGGCTACCTTGTTACGACTT-3') reported by Weisburg et al. The amplification was con ducted in (1991). The reaction was carried out in a final volume of 25 p:L containing approximately 150 ng of genomic DNA, 1X of reaction buffer (10 mM Tris-HCl pH 9.0, 50 mM KCl, 0.1% Triton X-100), 1.5 mM of MgCl2, 200 μM of each dNTP, 20 pMol of each oli gonucleotide and 2.6 units of the Taq DNA polymerase "expand high fidelity enzyme" (Roche®). The amplification profile consisted of an initial denaturation at 95 °C for 5 minutes, followed by 35 cycles: at 95 °C for 45 seconds, 58 °C for 45 seconds and 72 °C for 2 minutes. Finally, an extension at 72 °C for 10 minutes.
Identification of the Organic Acids by HPLC
The detection of organic acids was carried out by high-performance liquid chromatography (HPLC) in Promi nence LC20AT Shimadzu® with diode array detector SPD-M20A and column oven CTO-20-. The separation was carried out isocratically in a Phenomenex column of the reference Rezex™ (250 x 4.6 mm, 8 μm ROA-Organic Acid H+ (8%), separation mode by ion exclusion), using as a mobile phase H2SO4 (0.01 N) at a flow velocity of 0.2 mL-min-1 and temperature of 30 °C. The detection was carried out at a wavelength of 210 nm and the injection volume of the patterns and samples was 20 μL. The organic acids produced by the actinobacteria were identified by the comparison of the organic acid standards' retention times with the retention times of the chromatography peaks of each sample from filtrates of the fermentations from which the biomass had been previously eliminated.
Microorganisms and Culture
The phosphate solubilizing activity was assessed in growth curves under different sources of inorganic phosphorus. The isolation, screening and morphological identification of the actinobacteria and their selection as the best solubilizing isolation of phosphorus were carried out as reported by Salcedo (2014). The Streptomyces sp. T3A isolation presented high efficiency for the solubilization of phosphates from Ca3(PO4)2 and AlPO4 with values of 500 μg/ml and 25 μg/ml, respectively, which made it an interesting candidate for a more detailed characterization.
Evaluation of Streptomyces sp. T3A Under Different Sources of Inorganic Phosphorus
To assess the phosphorus solubilization activity of Streptomyces sp. T3A with multiple sources of inorganic phosphate (Ca3(PO4)2, AlPO4 and phosphate rock), an inoculant was prepared of 10 ml (10% VET, concentration 1 x 107 cells-ml-1), which was added to 90 ml of NBRIP medium supplemented with each source of insoluble phosphorus and taken to incubation at 23 °C and 120 rpm for seven days. Samples were taken every 24 hours during cultivation. The production of bio mass was measured by dry weight for each sample. The total culture volume (100 ml) was taken from the liquid fermentation. This volume of each culture medium with the different sources of phosphorus was subjected to centrifugation for 15 minutes at 5,000 rpm. The supernatant was separated and they were washed three times with saline solution (0.85%). The supernatant was discarded and finally, they were put inside an oven at 60 °C for three days, recording the weight of the tubes every 24 hours until a constant dry weight was reached. The substrate consumption was established by the DNS method and the soluble phosphorus released was established by the phosphomolybdenum method through the SPECTROQUANT test of Merck®. The pH was measured with a potentiometer (Miller, 1959; Murphy and Riley, 1962; Ramírez and Coha, 2003).
Evaluation of Streptomyces Sp. T3A Under Different pH Conditions
The inoculum corresponding to Streptomyces sp. T3A was added at 20 mL to the NBRIP medium supplement ed with tricalcium phosphate, aluminum phosphate and phosphate rock as a source of insoluble phosphorus, measuring an initial pH of 4, 5, 6 and 8 for each one of the treatments to evaluate. For each sample, the amount of soluble phosphorus released was established through the phosphomolybdenum method using the SPECTROQUANT test of Merck® (Murphy and Riley, 1962).
Results
Molecular Identification of Actinobacteria
The BLASTN analysis of the 15 sequences obtained showed that the isolations corresponded to the Streptomyces sp. genus. Table 1 presents the results of molecular identification and morphological characterization of the evaluated strains.
Identification of the Organic Acids Produced by the Isolations of Streptomyces Spp.
The evaluated strains were subject to the detection of organic acids by comparison with commercial standards. In Figure 1 shows the commercial standards of acids used and the presence of organic acids in the samples evaluated at the end of the fermentation time for the specific case of Streptomyces sp. T3A In some cases, it was possible to show the presence of un known compounds in the extracts, probably acids that were not within the standards used (Figure 1).
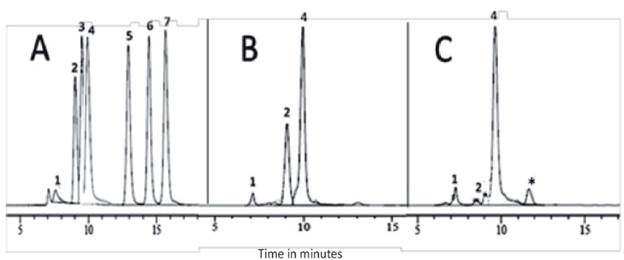
Figure 1 (A) Chromatography profile for the standards of organic acids: 1. Oxalic acid (7.15 minutes), 2. Citric acid (9.02 minutes), 3. Tartaric acid (9.49 minutes), 4. Gluconic acid (9.62 minutes), 5. Succinic acid (12.94 minutes), 6. Formic acid (14.45 minutes), 7. Acetic acid (15.71 minutes). * Unknown. (B) Chromatography of Streptomyces sp. (T3A) with Ca3(PO4)2 5 g • L-1 as source of phosphorus. (C) Chromatography of Streptomyces sp. (T3A) with AlPO4 1 g • L-1 as source of phosphorus
Oxalic, citric and gluconic acids were identified as the most predominant. It was also possible to observe that the intensity of the signals was different in the chromatographies with the different sources of inorganic phosphorus. Therefore, it can be deduced that the concentrations and type of organic acids produced by the actinobacteria also varied with the source of phosphorus to which the microorganism was exposed.
Table 2 presents the results of the isolations of all the studied localities in detail.
Table 2 Organic acids released by isolations in the fermentation trials with Ca3(PO4)2 and AlPO4 .
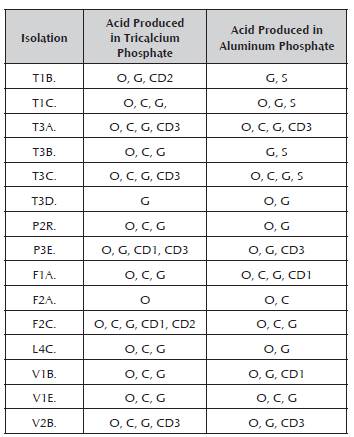
Oxalic acid (O), citric acid (C), tartaric acid (T), gluconic acid (G), succinic acid (S), formic acid (F), acetic acid (A), unknown com pound 1 (CD1), unknown compound 2 (CD2), unknown compound 3 (CD3).
Behavior of Streptomyces sp. (T3A) Under Different Sources of Insoluble Phosphorus
The behavior of Streptomyces sp. T3A under different sources of insoluble phosphorus (Ca3(PO4)2, AlPO4 and phosphate rock) is shown in Figure 2.
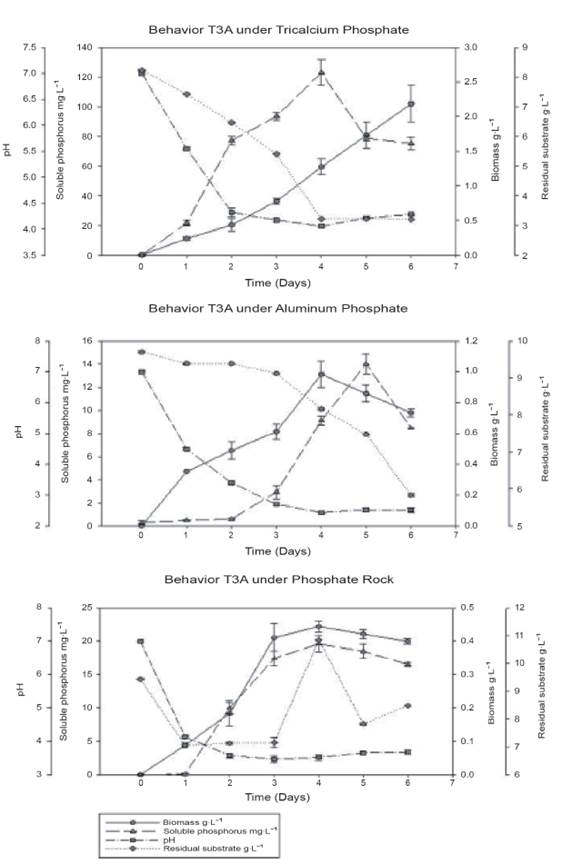
Figure 2 Behavior of Streptomyces sp. (T3A) under three different sources of inorganic phosphorus (a: Ca3(PO4)2, b: AIPO4, c: Phos phate rock).
Figure 2 shows that the soluble phosphorus released by Streptomyces sp. T3A with tricalcium phosphate as a source of phosphorus reaches a maximum of 122 mgP-L-1 on the fourth day of cultivation, and parallel to this, the lowest pH value was presented (4.05). Consequently, the behavior of the glucose presents a rapid decrease (8.22 to 3,21 g-L-1) during the first four days and later decreases slowly. From the fourth day, there is a decrease in the amount of soluble phosphorus that is released (78.7 mgP-L-1) and there is a significant increase in the production of biomass by the actinobacteria (1.2 to 2.2 g-L-1).
In the case of aluminum phosphate, it is observed that the maximum amount of soluble phosphorus re leased (14 mgP-L-1) is reached on the fifth day of incubation with a biomass concentration of 0.98 g-L-1, being a lower value if compared with the solubilization of tricalcium phosphate. It is also observed that the microorganism's glucose consumption was slower. With regards to pH, lower values were presented, de creasing to 2.3 and 2.5. It is also observed that there is a period of time in which although there are low pH values, forms of soluble phosphorus are not released into the medium. However, as an increase in glucose consumption is observed, parallel to this, the concentrations of soluble phosphorus increase.
Finally, the results observed during the trial with phosphate rock show that the maximum value of soluble phosphorus was obtained on the fourth day of growth, achieving a concentration of 19.6 mgP-L-1, which demonstrates the capacity of the actinobacteria to solubilize this kind of mineral source of phosphate.
Behavior of Streptomyces Sp. T3A Under Different pH Conditions
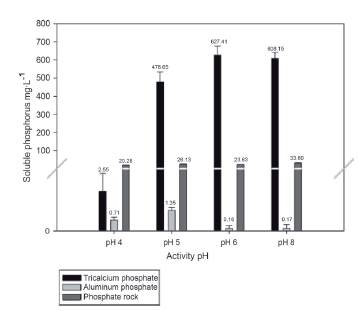
The solubilizing activity of phosphorus for Streptomy ces sp. T3A was assessed under different pH conditions (4, 5, 6 and 8) for the start of fermentation, with the aim to assess the ability of the actinobacteria to solubilize phosphorus in different pH ranges, taking into account that the soil microorganisms are mainly affected by different environmental conditions, including the pH of the soil. The results of the solubilization using tricalcium phosphate as a source of phosphorus are shown in Figure 3.
The results reveal that the solubilization of phosphorus as mentioned above is closely linked to the source of phosphates. However, in the case of this trial, it was possible to observe how in the medium with pH 4, there was a negative effect on the release of soluble phosphates compared to the other pHs evaluated, in which there is still good phosphate solubilization activity. This is an important characteristic taking into account that many soils for agricultural use in the country have an acidic pH and some are close to pH 8 (Burbano and Silva, 2010).
In terms of the results of phosphorus solubilization using phosphate rock as a source of insoluble phosphorus, a decrease is observed in the amounts of soluble phosphorus released when the microorganism is in pH 4 compared to the other pHs. Given that the characteristics of phosphate rocks can vary depending on their type, it is unclear in which pH the greatest solubilization activity of phosphorus can be established. However, for the kind of phosphate rock used in this work, the results obtained demonstrate the efficiency of the isolation of Streptomyces sp. T3A to act on traditionally used enhancers such as phosphate rock under an ample range of pHs.
Discussion
Molecular Identification of Actinobacteria
Based on an identity percentage of 99% or more, the identified isolations of actinobacteria belong to the Streptomyces spp. genus, a genus that is easily isolated due to its great adaptive capacity to different nutritional sources, which is related to its high population density in different kinds of soil, representing 1 to 20% of the total viable cell counts (Schrempf, 2001). Ad ditionally, isolation in simple organic media, such as the oatmeal medium or the ISP (International Strepto myces Project), promotes the presence of species of Streptomyces compared to other genera of actinobacteria, as a result of the secretion of a diversity of hydrolitic enzymes. This fact does not occur in culture media with complex structural sources (chitin, hydrolyzed animal hair and humic acid), which stimulate the growth of actinobacteria with slower growth rates, un like Streptomyces (Kurtböke et al. 1992; Seong et al. 2001; Hayakawa, 2008). The importance of the isolations belonging to this genus lies in the fact that due to their capacity to reproduce by conidia, to present filamentous growth and to produce a diversity of enzymes and secondary metabolites, they can adapt to different environmental conditions and thus maintain a high population, strongly adhere to substrates, break down a variety of compounds and compete for space and substrates compared to other groups of microorganisms. These characteristics make them good candidates for the production of biofertilizers involved in the release of soluble forms of phosphates (Waksman, 1950; Perlam, 1953; Goodfellow and Williams, 1983; Janssen, 2006; Zhang and Xu, 2008).
Identification of the Organic Acids Produced by the Isolations of Streptomyces Spp.
The fact that the presence of organic acids was detected in the different samples suggests that these com pounds are involved in the release of orthophosphates from insoluble sources. The capacity of the isolations to secrete acids into the medium may be due to the fact that the majority of the species of the actinobacterium phylum, despite being of an aerobic nature, maintain the genetic capacity of fermentation of their predecessors (Cellulomonas, Mycobacterium, Corine-bacteria), which supports their ample metabolic diver sity (Stackebrandt, 1991; Embley and Stackebrandt, 1994).
In terms of the reports of organic acids produced by this phylum, in subtropical soils, Chen et al. (2006) demonstrate the presence of gluconic acid and an unknown acid for different isolations of Rhodococcus erythropolis. Additionally, the presence of citric acid and two unknown acids was reported with strains of the Arthrobacter genus. In the work conducted by El-Tarabily et al. (2008), different genera of solubilizing actinobacteria of inorganic phosphates such as Micro-monospora, Nocardia, Actinomadura, Rhodococcus and Streptosporangium are reported. The solubilization of these bacteria was measured by acids such as oxalic acid, citric acid, malic acid, gluconic acid, acetic acid and lactic acid, as well as unknown acids.
Even when the results of this work propose the production of acids as the main release mechanisms of available forms of phosphates with in vitro trials, authors such as Hamdali et al. (2008, 2009, 2012) report that the solubilizing activity of actinobacteria is due to the presence of siderophores or chelating ions contrary to the organic acids, which cause alkalization of the culture medium. The results of the solubilization mechanism used by the actinobacteria of this research may vary with respect to those of Hamdali et al. (2008, 2009, 2012). This is reflected in the drop in pH generated during the fermentation processes (Figure 2). Furthermore, the results of this work present the production of organic acids as the main solubilization mechanism because no other kind of compound was shown that can cause the solubilizing effect when making an analysis with other wavelengths in the chromatography analyses (data not shown). Table 1 reports the presence of succinic acids, formic acid, acetic acid and the most predominant acids such as oxalic acid, citric acid and gluconic acid from the two sources of phosphate evaluated. These last three coincide with the bibliographic reports, where they are the ones that contribute the most to the release of phosphorus (Paredes-Mendoza and Espinosa-Victoria, 2009; Walpola and Yoon, 2012).
Behavior of Streptomyces Sp. T3A Under Different Sources of Inorganic Phosphorus
This document reports that the Streptomyces sp. T3A actinobacterium isolated from soils of the Eastern An des of Colombia is capable of solubilizing tricalcium phosphate, aluminum phosphate and phosphate rock as sources of insoluble phosphorus. According to the results presented in Figure 2 for tricalcium phosphate, there is an increase of soluble phosphorus in the medium while the pH decreases. The above may be explained by the greater production of organic acids that help to release the phosphorus from the insoluble in organic source. Additionally, the behavior of glucose supports this fact, suggesting that in the first days of fermentation, the glucose is directed at the release of organic acids in the culture medium. This process occurred simultaneously with the decrease in the amount of soluble phosphorus and an increase in the formation of biomass, which possibly occurs because the mi croorganism in the exponential phase requires greater consumption of nutrients, which may be limiting (in this case phosphorus). According to the above, it is possible to infer that excess of nutrients such as phos phates may be accumulated in the biomass (Franco, 2008; Hamdali et al. 2010; Chaudhry and Nautiyal, 2011).
The observations of the physiological behavior of Streptomyces sp. T3A toward aluminum phosphate (Figure 2) allowed a drop in pH to be shown from the moment of the inoculation of the microorganism, which is surely due to the release of the Al+3 ions, which acidify the medium. The results also show the directly proportional relationship in the increase in glucose consumption and the release of forms of soluble phosphorus, as a result of the production of organic acids synthesized by the microorganism. Finally, it is worth highlighting that the low amounts of soluble phosphorus released, the time in which they are reached and the growth rates of Streptomyces sp. T3A regarding the use of tri-calcium phosphate as a source of phosphorus suggest that the solubilization of aluminum phosphate occurs more slowly due to the stability of the complex and to the toxicity that it can cause in the microorganism, affecting solubilization. However, this in vitro test condition may cause different results with in vivo evaluations due to the fact that the phosphorus is in a different matrix, which influences its mobility generating other processes, such as immobilization, absorption or precipitation (Whitelaw, 2000; Oliveira et al. 2009).
The results presented for the solubilization of phosphate rock demonstrated the presence of solubilizing actinobacteria of phosphate rock and are comparable with the work presented by Hamdali et al. (2008), who investigated the solubilization of different kinds of phosphate rock in isolations of Streptomyces spp, which released amounts of soluble phosphorus in a range of 8.34 to 29.67 mgP-L-1. Contrary to the authors' findings in this work, the acidification of the medium as a result of the release of organic acids by consumption of the substrate was observed. This confirms that this is surely the solubilization mechanism that is be ingcarried out to solubilize phosphate rock. The high values of this element may be available in the medium or be stored by Streptomyces sp. T3A in the form of polyphosphates. Similarly, the physiological status of the strain, the culture conditions and other minerals present in the rock may alter the results of solubilization. Parallel to this, it is assumed that glucose consumption could not be established very accurately due to silicates or another kind of compound within the composition of the phosphate rock, which may interfere with the measurement of reducing sugars (Dighe et al. 1985; Hamdali et al. 2008; Schneider et al. 2010).
Behavior of Streptomyces Sp. T3A Under Different pH Conditions
The behavior that was observed for Streptomyces sp. T3A under pH 4 compared to the other evaluated pHs (Figure 3) may possibly be due to the fact that when adding acid to adjust the pH of the medium, it may be the acid that is carrying out the solubilization process. Therefore, when inoculating the microorganism, there are no insoluble forms of phosphorus so that it can develop its activity. On the contrary, it is possible that the actinobacteria make use of this for their growth. These results do not coincide with those found by Park et al. (2011), who evaluated the solubilizing activity of phosphorus of Burkholderia cepacia under different stress conditions, including the pH, finding that this microorganism is capable of solubilizing phosphorus in a pH range of 2 to 11. This fact also shows that not only is a drop in pH necessary to release soluble forms of phosphorus, but also the presence of organic acids produced by the microorganisms and that their solubilization scope depends on the type of organic acid, as well as the kind of soil (Liu et al. 2001; Park et al. 2011).
As observed in Figure 3, the solubilizing activity of aluminum phosphate is promoted in media with a low pH (4 and 5). This is probably due to the fact that the aluminum phosphates in aqueous solutions present mini mum solubility at high pHs. Therefore, when the pH decreases, the solubility of the phosphorus tends to increase, facilitating the availability of the compound so that the microorganism releases the available forms (Whitelaw, 1999; Sayed et al. 2000).
The results of Figure 3 present a similar trend to the research conducted by Vyas et al. (2007), who assessed the solubilization of phosphate rock in Eupenicillium parvum, demonstrating that as the microorganism is subjected to a stress condition (in this case a low pH), the phosphorus solubilizing activity is reduced. This typically occurs in fungi; microorganisms that metabolically have a similar metabolic behavior to actinobacteria. In addition to this, the differences in the results between studies may be attributed to the characteristics, composition and diverse forms of phosphorus (soluble and insoluble) that may occur in phosphate rocks, taking into account that when varying the pH, these may react differently, influencing solubility (Salas et al. 2006; Welch et al. 2002).
It is known that the addition of biofertilizers significantly increases the uptake of phosphorus by the plant. Therefore, the actinobacteria of this work may play a fundamental role in the solubilization of the phosphates present in soil, preventing the addition of more phosphorus, which is rapidly absorbed by the soil (Ahmed et al. 2008; Khan et al. 2009). Additionally, it is important to indicate that the isolation may present more activities that promote plant growth, which will finally contribute to a reduction in the use of other chemical compounds that harm the environment.
Conclusion
The findings of this study allow the suggestion of actinobacteria as microorganism with the phosphorus solubilizing phenotype; this being the first report of a solubilizing actinobacterium of aluminum phosphate. Finally, it is hoped that the results of this work lead to continuity of research on actinobacteria such as PGPR to be proposed in the design and formulation of biofertilizers in the agribusiness.











 text in
text in