Introduction
After nitrogen (N), phosphorus (P) is the essential nutrient for plant growth and development (Coyne, 2000). It plays a large role in protein synthesis, lipid biosynthesis, chlorophyll synthesis, carotenoid compounds and metabolisms of organic acids, and it intervenes in the biogenesis of carbohydrates in which it contributes energy in the form of ATP or ADP in the photosynthesis reaction that is important for many processes (Navarro and Navarro, 2003). However, soluble phosphorus is a limited nutrient for the production of biomass in a natural ecosystem (Hameeda et al. 2006).
A phosphorus deficiency in soil influences the harvest time and maturity of the plant's development, decreasing the yield of the crops. This leads to the implementation of chemical phosphatic fertilizers and consequently, phosphorus building up in insoluble and non-assimilable forms, because by adding it to the soil, the soluble phosphorus reacts with ions such as calcium, iron or aluminum causing its precipitation or fixation and reducing its availability for plants (Fernández et al. 2005).
Some microbial soil species have the capacity to convert the insoluble forms of phosphorus into assimilable forms for plants. The solubilizing action may be generated through processes such as the production of organic acids, chelation and exchange of reactions (Begonia et al. 2004). Biofertilizers are biological products based on microorganisms that live in the soil or in plants and that fulfill direct or indirect functions in their nutrition, by supplying, capturing or making essential elements available for the crops, as well as supplying substances that promote plant growth and induce systemic resistance to biotic or abiotic stress conditions (López, 2010). Biofertilizers or microbial inoculants constitute a vital component for agricultural ecosystems by mobilizing essential elements or making them more assimilable, like the case of phosphorus. Microorganisms that have demonstrated their efficiency in phosphate solubilization include several genera, such as Burkholderia cepacia, Pseudomonas sp., Aeromonahy drophilia, Pseudomonas luteola, Pseudomonas putida, Enterobacter sakasaki, Pantoea sp. and Enterobacter cloacae (Lara et al. 2011).
Biofertilizers based on rhizosphere microorganisms are an emerging alternative to inorganic chemical fertilizers for increasing the fertility and production of the crop in sustainable agricultural ecosystems, because they are products based on microorganisms with important functions in plant nutrition, they have a demonstrated beneficial effect on crops reducing the indiscriminate use of chemical substances and they improve the quantity and quality of internal resources (Echeverri and Castilla, 2008; Wu et al. 2005). Due to the above, the study of phosphate solubilizing microorganisms and the possible consortia that may occur between them is of great interest for increasing the availability of the element, and consequently reducing costs in the agricultural sector, one of the main bases of the economy of the Córdoba Department like other departments of Colombia.
As well as being agricultural, the Córdoba region is considered to be an important cattle area that provides the feed for animals, mainly with the existing fodder species, such as the Dichantium aristatum (Angleton grass). This plant is very appealing to cattle and it is used in hay manufacturing to supply the nutritional needs of animals in meat or milk production processes at a much lower cost than feed concentrate (Cuadrado et al. 2003). However, the cultivation areas of Angleton grass have been subject to the excessive use of chemical fertilizers, which has contributed to the ecological destabilization of the soil, having a negative effect on the microbial activity involved in plant nutrition. Consequently, the nutrients available to the plants have been limited (including phosphorus) making it a necessity to search for alternatives that can improve this panorama.
Knowing the importance of phosphorus for optimum plant development and taking into account the problem that low availability of P in the cultivation of Angle-ton grass implies, the phosphate solubilizing capacity of consortia formed by native bacteria of the Burkholderia cepacia, Pseudomonas sp., Pseudomonas luteola and Pantoea sp. genera was assessed with the aim to find the most efficient one to be used in the production of biofertilizers to apply to Angleton grass plants.
Materials and Methods
Antagonism Tests
The native strains Burkholderia cepacia, Pseudomonas sp., Pseudomonas luteola and Pantoea sp. were used, isolated from rhizosphere soils of cultivable areas of the Córdoba Department, Colombia, which demonstrated their efficiency in their phosphate solubilizing capacity and that belong to the bank of strains of the GRUBIODEQ Biotechnology Laboratory (Universidad de Córdoba) (Lara et al. 2011). The strains were subjected to antagonism tests with the aim to verify that there was no inhibition between them, and therefore, that they would be able to form the consortia. The antagonism tests were carried out in Mueller Hinton agar through the technique of diffusion in agar (Beltrán et al. 2005). The criterion of elimination of strains was established by the presence of halos of growth inhibition with diameters of more than 5 mm between the massively planted strain and the opposing strain (Barragán et al. 2003).
Qualitative and Quantitative Tests
To conduct the qualitative and quantitative tests, bio-preparations of each strain were developed using 100 mL Erlenmeyer flasks that contained 45 mL of sterile culture medium and taking into account the conditions of microbial growth: environmental temperature (28±2 °C), incubation time of 12-18 hours and constant agitation of 150 rpm. Biopreparations of concentrations of 108,107 and 106 CFU/mL were prepared, which were quantified through serial dilutions by the microdrop technique (Moreno et al. 2000). Later, the qualitative and quantitative assessment of the phosphate solubilizing capacity of the native strains was conducted, individually as well as in consortia, at the concentrations of 108, 107 and 106 CFU/mL. The consortia were formed with isolated strains that did not present any antagonistic effect at equal concentrations.
Qualitative Assessment of Phosphate Solubilization
The qualitative phosphate solubilizing capacity presented by the microorganisms at concentrations of 108,107 and 106 CFU/mL was established in the NBRIP medium for biopreparations prepared with pure strains individually and in consortia (Nautiyal, 1999). After being inoculated by the well technique, the NBRIP Petri dishes with each one of the pure strains and with the bacterial consortium were incubated at 28 °C for 14 days until clear halos of the CFUs of phosphate solubilizers appeared. The size of the halos was calculated according to the solubilization index: SI=A/B (A: diameter of the colony + halo diameter and B: diameter of the colony) (Kumar and Narula, 1999). The measurements were made at 7 and 14 days after inoculation, respectively, with the aim to assess the behavior of the strains over time. All of the tests were conducted three times.
Quantitative Assessment of Phosphate Solubilization
The quantitative capacity of phosphate solubilization was determined in NBRIP (liquid) medium. The vana-domolybdophosphoric acid colorimetric method was used (Kitson and Mellon, 1944) based on a diluted solution of orthophosphate. The ammonium molybdate reacts in acidic conditions to form a heteropoly acid, phosphomolybdic acid. In the presence of vanadium in the form of yellow vanadomolybdophosphoric acid, the intensity of the yellow color is proportional to the concentration of phosphates. Using this method, bacteria were evaluated that have the capacity to solubilize inorganic, insoluble phosphate components, such as tricalcium phosphate, dicalcium phosphate and hydroxylapatites. For this test, 1 mL of biopreparation was taken and it was added to 9 mL of sterile NBRIP medium, then it was incubated for 72 hours at room temperature (28±2 °C) in constant agitation at 150 rpm. After this time had passed, the supernatant was taken, the reactive (vanadomolybdate) was applied and the absorbency at the wavelength of 440 nm was read using a Genesys 20 Vis spectrophotometer (Thermo Fisher Scientific Inc.) and using the calibration curve: y = 0.0059x + 0.0037, R2 = 0.9912, standardized by the research team of the GRUBIODEQ Laboratory. The concentrations were established in ppm. As a target, only the NBRIP medium was taken and all of the process was carried out. All of the tests were conducted three times.
Evaluation of the Effect of Bioinoculants on Angleton Grass Plants (Dichantium Aristatum)
Once the efficient microorganisms or consortia were selected, they were planted by the mass streaking technique in nutrient agar and incubated for 24 hours at 36 °C. The bioinoculants were produced in 100 mL Erlenmeyer flasks with 30 mL of sterile nutrient broth. Each Erlenmeyer flask was inoculated with the efficient microorganisms and incubated at room temperature (28±2 °C), maintaining constant agitation of 150 rpm in a shaker for a period of 24 hours. With the aim to confirm the cellular concentration in each bioinoculant, the growth was measured by the serial dilution method at the end of the process. All of the tests were conducted three times (Seeley et al. 1973). In this way, the inoculants that presented a greater yield in phosphate solubilization were adjusted to the concentration.
To conduct the experiment, Angleton grass (Dichantium aristatum) seeds were used disinfected with 70% ethanol for 5 minutes and later with 3% sodium hypochlorite solution for 5 minutes. The seeds were immersed in the bioinoculants for 60 minutes and were assessed under controlled nursery conditions. For this, a completely randomized design (CRD) was used with three treatments and five repetitions, which consisted of:
Treatment 1 (control): Seeds with no kind of treatment.
Treatment 2: Seeds treated with the bioinoculant in the most effective concentration and without chemical fertilization.
Treatment 3: Seeds treated with the commercial chemical fertilizers diammonium phosphate (DAP) and urea.
The soil used presented the following characteristics: MO = 1.38%; P = 7.5 ppm, DA = 1.5 g/cm3 and K = 0.88 mEq/ 100 g). The chemical fertilization was conducted according to the soil analysis and the nutritional requirements for Angleton grass (N: 230; P: 53; K: 252 ha/year). According to the above, 0.9 g/ recp of urea and 0.0144 g/recp of DAP was applied to the treatment with chemical fertilizer at eight days after planting (DDS). The experiment was conducted in plant pots with an area of 0.045 m2.
For each plant pot, 2 g of seeds were used, which were planted by scattering at 1 cm deep and randomly throughout the surface of the plant pot. The test was conducted on facilities of the Universidad de Córdoba, (3 Km. vía Montería-Cereté) in a nursery of the Agronomic Engineering Department with an average temperature of 29 °C, atmospheric pressure of 756.96 mm and relative humidity of 79%. The soil used had a clay texture taken from areas of the Universidad de Córdoba (Degiovanni et al. 2004) without previous cultivars. No type of pesticide was used. Measurements were taken at 21 and 35 days after germination. In each sample, three plants were collected at random from each repetition. The variables to take into account were the following biometric parameters (Hernández, 2002 and Ramírez and Pérez, 2006): a) number of leaves (No.): count of cotyledon and truly photosynthetically active leaves in the different plants; b) leaf area (cm2): measured taking as a reference the millimeter squares of sheets of paper in each one of the cotyledon leaves of the sampled plants; c) plant length (cm): measurement from the root to the largest leaf of each one of the sampled plants; d) root length (cm): measurement of the main root of each sampled plant; e) dry weight: dried on a stove at 60 °C until a constant dry weight was reached. Once dry, their weight was determined. All of the tests were conducted three times.
Statistical Analysis
The data obtained from the biometric parameters of the Angleton grass plants were assessed using the SAS 9.2 (SAS, 2010) statistical package. A completely randomized design was used and the means were compared through orthogonal contrasts in the same software. P < 0.05 and p < 0.01 were considered to be significant differences and highly significant differences, respectively. Tukey's test was also applied.
Results and Discussion
Antagonism Test
When taking the reading, antagonism was not shown by any part of the strains, since they developed without inhibiting growth between them. The above indicates that there is no kind of antibiosis, competition for space or nutrients, or direct interactions with the pathogen (enzymatic lysis) that obstruct the free development of the microorganisms.
Qualitative Assessment of Phosphate Solubilizing Capacity
The measurements of the qualitative phosphate solubilizing capacity taken at 7 and 14 days individually and in consortia are shown in Table 1 and Table 2.
Table 1 Average solubilization indexes at 7 days.
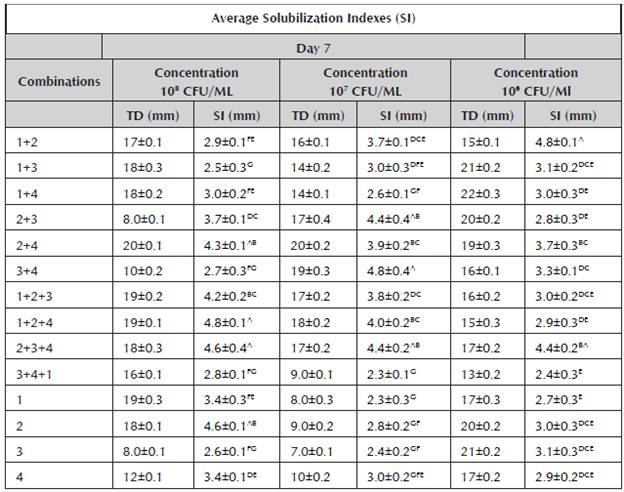
1.Burkholderia cepacia, 2. Pantoea sp., 3.Pseudomona luteola, 4. Pseudomona sp., solubilization indexes (SI), average total diameters of the colonies (TD), a. Arithmetic means ± standard deviation of experiments repeated three times. The values followed by different letters in superscript indicate significant differences (p < 0.05) according to Tukey's test.
Table 2 Average solubilization indexes at 14 days.
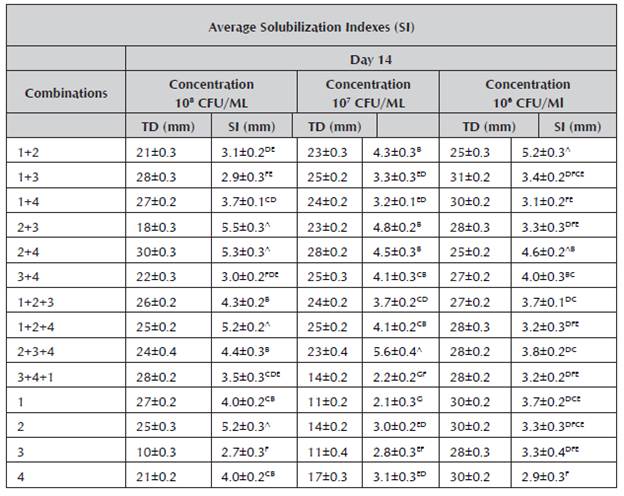
1.Burkholderia cepacia, 2. Pantoea sp., 3.Pseudomona luteola, 4. Pseudomona sp., solubilization indexes (SI), average total diameters of the colonies (TD), a. Arithmetic means ± standard deviation of experiments repeated three times. The values followed by different letters in superscript indicate significant differences (p < 0.05) according to Tukey's test.
On the seventh day of the test, it was observed that the average values of the solubilization indexes oscillated between 2.3 and 4.8 mm (Table 1). The following consortia stood out in this stage: Burkholderia cepacia + Pantoea sp. + Pseudomona sp. at the concentration of 108 CFU/mL, Pseudomona luteola + Pseudomona sp. at the concentration of 107 CFU/mL and Burkholderia cepacia + Pantoea sp. at the concentration of 106 CFU/ mL. The above demonstrates that the consortium formation represents greater individual solubilization, exceeding that of the findings of Lara et al. (2011a) with a SI of 4.0 mm, and similar to the findings of Bobadilla and Rincón (2008) with a SI from 3.3 - 5 mm.
Table 2 summarizes the average values of the solubilization indexes of the strains individually and in consortia on Day 14 of test. The values oscillated between 5.6 and 2.1 mm.
At 14 days, a similar trend was observed in the individual strains and those in combination to the one obtained at seven days. The consortia were highlighted again, including Pantoea sp. + Pseudomona luteola, Pantoea sp. + Pseudomona sp. and Pantoea sp. + Pseudomona luteola + Pseudomona sp. The latter presented the highest values in the solubilization indexes with 5.6 mm, results that exceeded those found by Guzmán (2012), where the greatest solubilization index at 14 days was 5.05.
The test results demonstrated that the phosphate solubilization indexes of some consortia were greater in comparison to the SI of the individual strains, demonstrating a favorable synergic effect.
Quantitative Assessment of Phosphate Solubilizing Capacity
The data obtained from the quantitative assessment of the phosphate solubilizing capacity of the native strains using the vanadomolybdophosphoric acid colorimetric method is summarized in Table 3. Values between 117 and 842 ppm are observed.
Table 3 Average concentrations in parts per million (ppm) of phosphate solubilization.
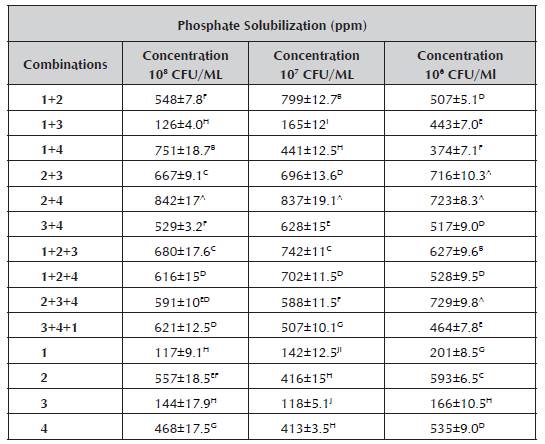
1. Burkholderia cepacia, 2. Pantoea sp., 3.Pseudomona luteola, 4. Pseudomona sp., Concentration in parts per million ([] in PPM), a. Arithmetic means i standard deviation of experiments repeated three times. The values followed by different letters in superscript indicate significant differences (p < 0.05) according to Tukey's test.
The microorganisms in consortia presented greater phosphate solubilization than those that were found individually, due to the fact that the microbial consortia in many cases interact synergically, stimulating some physical or biochemical processes of the bacteria involved in the combinations. The concentration at which greater efficiency occurred was 108 CFU/mL, highlighting the consortium comprised of Pantoea sp. + Pseudomona sp. Additionally, the same showed good results in the concentrations of 107 and 106 CFU/mL. Also in this stage, the consortium comprised of Burk-holderia cepacia + Pantoea sp. at the concentration of 107 CFU/mL stands out.
The results above exceed those found by Cordero et al. (2008) in the evaluation of a strain of Pantoea sp. (9C), which presented solubilization of approximately 400 μg P mL-1 during 40-60 hours. Similarly, studies conducted by Osorio and Lara (2013) were exceeded, who evaluated the consortia formed by Pantoea sp. and Azotobacter sp., obtaining results of 602.60 ppm at the concentration of 106 CFU/mL.
The results of the quantitative assessment confirmed that the consortium formed by Pantoea sp. + Pseudomona sp. at the concentration of 108 CFU/mL was the best, corroborating the results obtained in the qualitative assessment. Due to the above, the consortium was selected for the following test.
Tests on Angleton Grass (Dichantium Aristatum) Seeds
Table 4 shows the average results of the biometric parameters measured corresponding to the amount of leaves, plant length, leaf area, length of main root and dry weight for the different treatments.
Table 4 Effect of the bioinoculants on the growth and development of Angleton grass (Dichantium aristatum) plants.
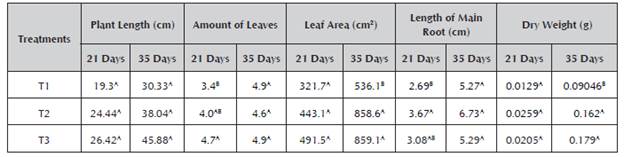
A,B Same letters do not present significant differences with an alpha of 0.05. The data represent the average of five repetitions. The statistical comparisons were carried out independently for each time in the different variables. Treatment 1 (control): Seeds with no kind of treatment; Treatment 2: Seeds treated with the consortium Pantoea sp + Pseudomona sp at the concentration of 108 CFU/mL; Treatment 3: Seeds treated with commercial fertilizers diammonium phosphate (DAP) and urea.
Plant length (cm): Statistically, significant differences did not occur between the treatments at Days 21 and 35. However, greater length was shown for T3 and T2 (45 and 38 cm, respectively) compared to T1. T2 (consortium) showed a greater value than the control (T1), which may be due to the availability of the phosphorus provided by the consortium, taking into account that this nutrient is essential for the plants' growth and development (Coyne, 2000). It is also possible that the strains may produce plant hormones promoting plant growth, because Pseudomonas sp. is found within the bacterial genera that have been reported as producers of IAA (Ahmad et al. 2006). The data obtained are in agreement with the research conducted by Rajkumar et al. (2006), who reported an increase in the length of mustard plants inoculated with Pseudomonas sp and Bacillus sp compared to the plants that were not inoculated. Similar results were obtained in the work of Galvis and Lara (2013) using Angleton grass plants.
Amount of leaves (no.): For this biometric variable, the best average results for T3 were observed at 21 days, showing significant differences (p < 0.05). These data may be attributed to the greater availability of nutrients provided by chemical fertilization. Significant differences were not presented at 35 days among the average results of the treatments. This is in line with studies conducted by Pastor and Lara (2013), who for the number of leaves variable in Angleton grass did not find significant differences in any of the measurements taken.
Leaf area (cm2): Greater leaf area was shown in T3 and T2 compared to T1, obtaining a highly significant difference (p < 0.01) at 35 days. This characteristic leads to a better photosynthetic capacity of plants, which results in greater growth and greater development of the leaf area (Mayak et al. 2004; Santillana, 2006). Research conducted by Ahmad et al. (2013) has found a correlation between the increase in the leaf area by the joint inoculation of rhizobacteria of the Rhizobium and Pseudomonas genera and the increase in the chlorophyll content in mung bean plants (Vigna radiata L.).
It is important to highlight that the effect of the inoculated treatment (T2) on the leaf area was similar to the treatment with chemical fertilizer (T3), demonstrating the benefits of the native consortium.
Root length (cm): The results for this parameter at 21 days show a significant difference (p < 0.05) in root length, because there was a difference between T2 (inoculated seeds) compared to T1 (control, seeds with no kind of treatment). T3 (seeds treated with commercial fertilizers) was similar to the two other treatments. At 35 days, T2 achieved greater root length with a value of more than 6 cm, while T1 and T3 showed a length of around 5.27 cm. A significant difference was not presented. The consortium demonstrated positive effects on root length, which may be attributed to the availability of phosphorus and also to the possible presence of plant hormones produced by one or both strains that form the consortium. The growth promotion has been attributed to factors such as the synthesis of certain plant hormones, which stimulate the density and length of the radical hairs, thus increasing the amount of roots in the plants, increasing the capacity for the absorption of water and nutrients and allowing the plants to be stronger, more productive and more tolerant of adverse climatic conditions ( Lara et al. 2011b; Kloepper et al. 1991).
Dry weight (g): The highest dry weights were shown in T3 and T2, achieving a maximum weight of 0.179 g. A lower value was shown in T1 with a weight of around 0.09 g. At 35 days, a highly significant difference (p < 0.01) was observed between T2 and T3 compared to the control (T1), which presented a low dry weight. The assimilation of phosphates by the plants contributes to an increase in their metabolism, which is reflected in a greater content of organic material, growth of roots as a seedling, acceleration of maturity, stimulation of coloring of the fruits and supported formation of seeds and of the energy transfer molecules such as ATP (Madigan et al. 2003; lanez, 2010).
The presence of plant organs with a net demand by assimilates may strongly influence the production and distribution patterns of dry material (Tekalign and Hammes, 2005). The accumulation of dry material is commonly used as a parameter to characterize growth, because it usually has great economic significance.
Conclusions
The microorganisms in microbial consortia demonstrated a greater phosphate solubilizing capacity.
The application of bioinoculants at the base of native, phosphate solubilizing microbial consortia of Angleton grass (Dichantium aristatum) represents a notable benefit in diverse biometric parameters, such as leaf area, root length and dry weight.
The results obtained represent a great alternative for the partial substitution of chemical fertilizers with good results for the growth of Angleton grass plants at a low cost and in an environmentally-friendly way for cleaner production.











 texto em
texto em 


