Introduction
Light availability and a light/darkness system are considered to be two of the most important factors that limit the productivity of microalgal biomass in environmental conditions (Richmond, 1990). The turbulent flow in the culture represents a practical medium to improve the distribution of solar energy. When turbulence is insufficient it becomes the pattern for laminar flow, resulting in a decrease in the effectiveness of solar use. Turbulence also increases the rates of exchange of nutrients and metabolites between cells and their growth environment (Grobbelaar, 1994). It has been demonstrated that by increasing the turbulent flow of Spirulina cultures in ponds, productivity increases, as well as the optimum density (Richmond and Vonshak 1978; Vonshak et al. 1982).
The selection of an optimum design of photobiore-actor for the mass cultivation of microalgae is an important factor that governs general photosynthetic productivity. To date, almost all commercial production of this alga is carried out in ponds in which the culture is agitated with a paddle wheel. Closed photo-bioreactors provide many advantages, such as greater availability of light (high surface to volume ratio), temperature control, high concentration of biomass, decrease in harvest costs, low rate of contamination and ease of automating the process with the aim to achieve greater use of solar energy and temperature at all times. Therefore, the purpose of the design and development of the photobioreactors in environmental conditions is to use sunlight, reduce costs and achieve maximum algae production. In relatively warm regions, where the majority of algal biomass is produced, it has been assumed that the main limiting factor for growth is light. Many studies have tried to increase the light available for the algal cells cultivated in environmental conditions, while Richmond and Vonshak (1978) demonstrated an increase in the productivity of cultures of A. platensis in environmental conditions by increasing the turbulent flow. This observation was confirmed by later studies (Laws et al. 1988; Fontes et. al. 1991; Grobbelaar, 1994; Sukenik et al. 2009). The explanation of this phenomenon continues to be the subject of debate. Some of the explanations have attributed the result to the improvement in the transfer of nutrient mass, the effect of flickering light (Kok, 1956) and the elimination of excess oxygen (Torzillo et al. 1998). However, it is generally accepted that the increase in turbulent flow in algal cultures in the open air improves the light/darkness cycle and the cultures' photosynthetic efficiency (Richmond, 2004). The mass production of Arthrospira in ponds has an estimated annual production of 8,000 metric tons. Greater development of this industry is dependent on the capacity to reduce the production costs to allow the commercialization of Arthrospira. A way of achieving this objective is by increasing the productivity of the cultures in environmental conditions by overcoming limiting environmental factors, such as temperature and light. Algal cultures in environmental conditions are exposed to daily fluctuations of light, which not only limit the light, but also cause photoinhibition of the culture for a significant part of the day (Vonshak and Guy, 1992; Lu and Vonshak, 1999). Although solar radiation occurs in environmental conditions, the light available can be modified for algal cells cultivated using different methods.
In Peru, the desert region of Arequipa presents great solar illuminance characterized by a clear sky and the absence of rain throughout the whole year, as well as a high daily temperature adequate for biomass production. However, with improvements, the photobio-reactor design could capture almost all the solar light available. The objective of this study is to assess the productivity of Arthrospira platensis used in mass cultivation in a doubly curved tubular photobioreactor under environmental conditions, observing the morphological changes in the filaments of A. platensis.
Materials and Methods
Organism and Culture Conditions
The inoculum of A. platensis was obtained from the Biotechnology Microalgae Collection of the Aquatic Biology Laboratory -UNSA, Arequipa, Peru. The culture was maintained using the "La Molina" hydroponic solution at 25% (Rodriguez-Delfin et al. 2001) with the following composition (g/L): Solution A: Triple Superphosphate 35; KNO3 110; NH4NO3 70; and Solution B: MgSO4 44; Fetrilon Combi 2.5; boric acid 0.24 and NaHCO3 8 g (Merck Brand).
All of the nutrients were dissolved in dechlorinated water, adjusted to pH 9 and sterilized at 120 OC and 15 Lb of pressure for 20 minutes. The volume of the A. platensis culture in the photobioreactor was 5.73 L, maintaining the pH range between 9-10 and with a maximum temperature of 22.6 OC and a minimum temperature of 9.4 OC. The culture in the open air in photobioreactor was located at 16O24'50" south latitude, 71O32'02'' west latitude and at an altitude of 2,344 m.a.s.l.
Design and Operation of the System
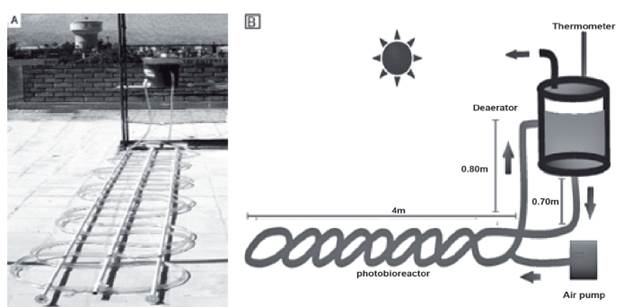
The system used in this study consisted of three parts: (1) Doubly curved tubular photobioreactor, measuring 4 m in length, built with a transparent polyvinyl chloride (PVC) tube, measuring 15.3 m in length and with an internal diameter of 12 mm; 2) Deaerator with a capacity of 4 L; 3) Air pump, ACQ-001 model; Electromagnetic Air Compress of the BOYU Company (Figure 1) (Carlozzi and Torzillo, 1995). The photobioreactor was covered with an anti-UV sheet and 50% Raschel mesh to prevent the excessive UV radiation caused by direct exposure to sunlight. An air pump was used for the mixing and aeration of the microalgae. The PVC tube that forms the photobioreactor occupies a surface area of 0.577 m2. This value was used to calculate the production of biomass per square meter The total volume of the tube that forms the photobioreactor was 1.73 L = (ω x (0.006 m)2 x 15.3 m). The proportion between the volume and the illuminated surface area (PVC tube) of the photobioreactor was 0.33 m2/L. The total volume of the system was 5.73 L, which is calculated by adding together the volumes of the photobio-reactor and the deaerator.
Solar Radiation and Experimental Conditions
The solar radiation was measured with a solar UV radiometer, Davis brand, 06490 model, and the illuminance was measured with a digital light meter, Extech brand, Model 401025, measured by the Meteorological Station of the Professional School of Physics of the UNSA, Arequipa, Peru. The experiment was conducted from October 18 to November 1, 2013.
Morphological Observation of the Filaments of Arthrospira Platensis
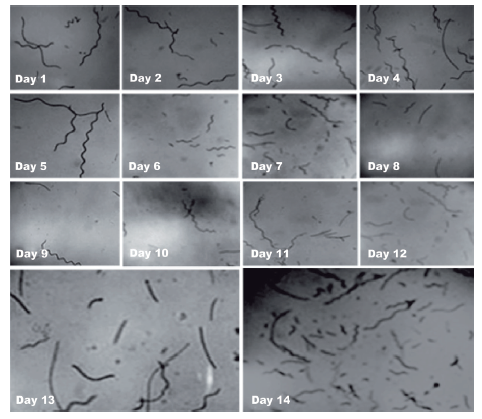
The morphological changes of A. platensis filaments were examined using an optical microscope (Labor-Tech, MODEL: 2001, Germany). The digital images were recorded with a digital camera, observing the morphology of the filaments daily (Figure 2).
For the ultramicroscopic comparison of the filaments of A. platensis, a scanning electron microscope was used, Philips brand, SEM XL 20 with an energy-dispersive X-ray microanalysis, EDAX DX 4i brand. The samples were covered with a metallic layer of gold approximately 100 A° thick with the Denton brand Vacuum Desk II metalizer (Figure 3).
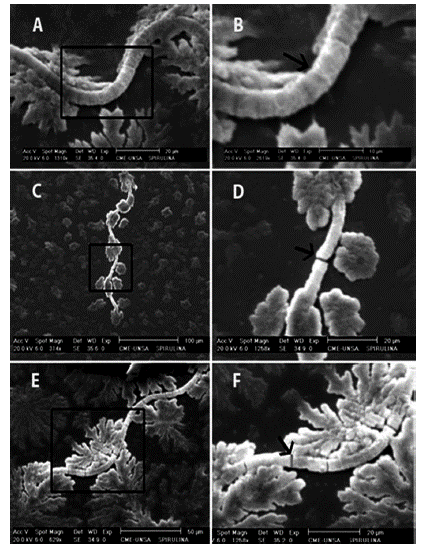
Figure 3 Comparison of the microscopic structure of Arthrospira platensis, which shows the morphological variation of the filaments. (A, B) - View of a normal filament. (C, D, E, F) - View of fragmented filaments. The trichomes of the fragmented filaments are indicated with arrows (Photos taken by J. Soto. Electron Microscopy Center (CME), Universidad Nacional de San Agustín (UNSA), Arequipa - Peru).
Growth of A. Platensis
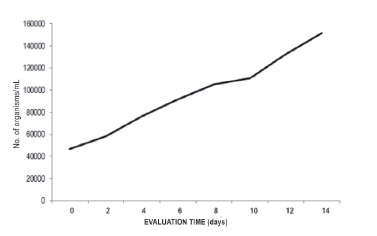
The growth of A. platensis was developed in a doubly curved tubular photobioreactor with 5.73 L of culture at an initial concentration of 48x103 org/ml. The filaments were counted in a Sedgwick-Rafter Counting Chamber (Moheimani et al. 2013).
Establishing the Biomass
To establish the content of chlorophyll "a", the samples of A. platensis were concentrated by centrifugation at 10,000 rpm for ten minutes and they were lysed with liquid nitrogen. The optical density was measured by the spectrophotometric method at 750 nm and 664 nm (APHA, 1992). The dry biomass was established using a stove for two hours at 105 OC (Vonshak, 2002).
Results and Discussion
Solar Radiation and Experimental Conditions
A mean solar radiation of 953.6 W/m2 was obtained and the maximum radiation was achieved after Day 10 at 1179 W/m2. The upper limit was 1143 W/m2 and the lower limit was 764.01 W/m2 (Figure 4). Due to the geographical location of Arequipa, the days have high solar radiation with variable and low temperatures at night for most of the year. The measures of open air illuminance were 121,500 lux.
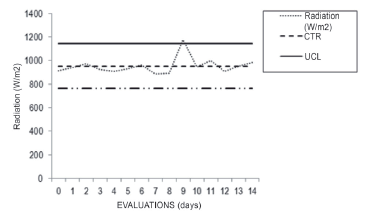
Figure 4 Maximum solar radiation taken from October 18 to November 1, 2013. (Source: Meteorological Station of the Professional School of Physics (EPF) of UNSA)
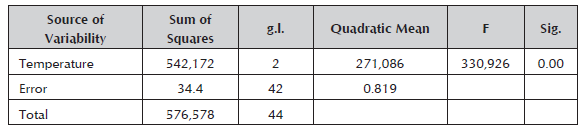
A. platensis in a doubly curved tubular photobioreactor had an average speed of the culture medium of 0.3 m/s with an average flow of 0.034 L/s. The flow of the air pump without culture medium was 0.0833 L/s. The ANOVA comparison shows the Fisher statistic (F = 330.926) with highly significant differences (p < 0.01) in the four points of measurement of temperature: 1) in the culture in photobioreactor; 2) in the external environment; 3) within the system; and 4) measurements taken in the Meteorological Station of the Professional School of Physics (EPF, for the Spanish original) of UNSA (Table 1). The measured temperature reached was 25.74 °C as an upper limit and the mean temperature was 24.28 °C up to Day 14 of the evaluation and 22.82 °C as a lower limit at the 95% confidence level (Figure 5). Tukey's test showed a greater record in T° (culture) with 24.27 + 0.66 °C; while the lowest record was presented in T° (open air) (Meteorological Station EPF) with 16.46 + 0.97°C (Figure 6).
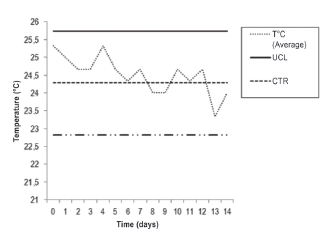
Figure 5 Ratio between the mean temperature of production of Arthrospira platensis in a doubly curved tubular photobioreactor and assessment time (in days). UCL = Upper Confidence Limit; LCL = Lower Confidence Limit; CTR = Mean temperature value.
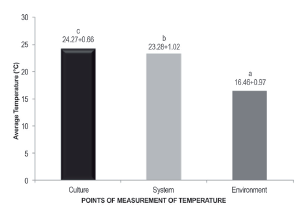
Figure 6 Tukey's multiple comparison test for the temperature measurement points in a doubly curved tubular photo-bioreactor.
Morphological Changes of Arthrospira Platensis
The filaments of A. platensis cultivated in photobio-reactor in the first five days of cultivation maintained their characteristic spiral shape (up to 13 coils) with the appearance of the first fragmentations in the filaments. On Day 6, an increase was observed in the number of fragmentations. On Day 7, very long filaments were observed with scarce formation of coils (Figures 2 and 3C). On Days 8 to 14 the proportion of fragmented filaments increased.
The ruptures of the filaments of the spiral structure of A. platensis were studied by Wu et al. (2005), who mentioned that the ruptures are altered by the low temperatures and not by the natural levels of ultraviolet (UV) radiation. Furthermore, many cyanobacteria are capable of synthesizing absorbent UV compounds in response to excessive radiation, such as mycosporines, amino acids and scytonemin cells that screen the cells before UV radiation reaches the intracellular components (Garcia and Castenholz, 1993). Therefore, in this study, the rupture of the filaments may be due to low temperatures of the area at night and at dawn (15-18 °C), which suggests that these morphological changes are connected to physiological and molecular processes at low temperatures and not in response to excessive UV radiation. In studies by Vonshak et al. (1994) and Vonshak (1997), it was found that the low morning temperature together with the high light intensity could induce photoinhibitory stress on A. platensis and that increasing the temperature of the culture to 35 °C may reverse the inhibition and increase the daily production almost fourfold.
Throughout the research culture, it was observed that the spiral filaments of A. platensis are longer and they are a lighter green color than those cultivated under laboratory conditions. One possible cause of the greater length of the spiral filaments of A. platensis could be their spiral configuration, due to the fact that the filaments adopt a tighter helicoidal structure with small coils allowing greater length. The strains of A. platensis with a tighter helicoidal structure can tolerate greater light intensity compared to the strains with more flexible spirals (Jeeji and Seshadri, 1980). Furthermore, loose spirals could transform into tighter spiral shapes when changed to intense light conditions (Fox, 1996; Wu et al. 2005), working with two different strains of A. platensis. This suggests that the transition of loose coils to very tight coils in the presence of UV-B radiation could be an effective protection mechanism. This self-shading morphological change of A. platensis due to the tighter spirals seems to be associated with this organism's protection strategy to counteract solar (UV) radiation. It is well known that UV radiation leads to damage, such as whitening of the pigments, degradation of proteins and enzyme inactivity in many organisms. However, some organisms have developed repair strategies against it, such as de novo protein synthesis (for example proteins D1 and D2) (Sass et al. 1997), repair of DNA (Britt, 1995; Hader and Sinha, 2005) and the elimination of oxygen radicals (Mittler and Tel-Or 1991; Middleton and Teramura, 1993).
In the study of the ultra-microscopic structure of filaments of A. platensis using electron microscopy at different scales (20, 100 and 50 μm), a decrease is observed in the number of coils in the filaments of A. platensis (Figures 3C and 3D) with fragmentation, as well as the observation of filaments with several points from fragmentation, indicated with arrows (Figures 3E and 3F).
Biomass Productivity
The concentration of chlorophyll "a" in biomass values in a doubly curved photobioreactor was 0.00785 mg/L, with illuminance of 121,500 lux. The composition of chlorophyll "a" present in Spirulina varies from 0.8 to 1.5% of dry material (Paoletti et al. 1980). According to the count of filaments of A. platensis, the maximum growth was obtained at 14 days with 151,667 org/ mL, with growth that is adjusted to the "linear" model (Y=a + bX) in a doubly curved tubular photobioreactor (R2=0.990; F=593.060) (Figure 7) (Guisande et al. 2013).
A biomass concentration of 1.4 g/L was obtained at the end of the 14 days with a system of 5.73 L; a total production of 8.022 g obtaining productivity of 13.9 g/m2 or 1 g/m2/day in 14 days at 121,500 lux in the doubly curved tubular photobioreactor. A lot of research has been conducted on A. platensis cultivated in photobio-reactors in environmental conditions by Vonshak et al. (1996), where concentrations between 2 to 2.225 g/L of dry biomass were obtained in 24 hours with total solar irradiation of 254.6 W/m2. Andrade and Costa (2008) produced cultures of Spirulina platensis in the open air in the far south of Brazil, obtaining 1.94 g/L with a maximum productivity of 0.059 g/L/day. Other authors such as Zittelli et al. (1996) obtained average productivities of 0.83, 0.44 and 0.61 g dry weight L-1 d-1 in fall (September-October), winter (November-December) and March, respectively.
The results present the productivity achieved under the environmental conditions of the Arequipa region, characterized by high solar radiation and very variable temperatures of 22.6 °C to 9.4 °C, lower than the results obtained by Vonshak et al. (1996) and Zittelli et al. (1996). This low biomass production could be due to the effect of photoinhibition because of the high radiation of the region. Although photoinhibition has been intensely studied over the last ten years (Baker and Bowyer, 1994; Kyle et al. 1987), there has been very little study of the effect of productivity on the culture systems of microalgae. The role of photoinhibition as an ecological factor in aquatic systems has been reviewed by Neale (1988), who concludes that the importance of photoinhibition on aquatic systems is growing and it is considered as a factor in the estimation of aquatic productivity. Studies on photoinhibition in algae of commercial interest are very limited, with Vonshak et al. (1988) being the first to study photoinhibition in Spirulina under laboratory conditions followed by a later study to estimate the effect under external conditions.
Vonshak and Guy (1992) quantified the effect of photoinhibition on productivity, estimating that the loss of the production potential of cultures in open systems may be more than 25% due to photoinhibition. The interaction of stress from light and temperature was studied by Jensen and Knutsen (1993), who demonstrated that a culture temperature below the optimum one for growth of Spirulina is more sensitive to photoinhibition. Furthermore, Vonshak et al. (1994) demonstrated that photoinhibition in tubular photo-bioreactors under environmental conditions may be prevented by increasing the temperature to above the optimum temperature during the day. Much research is needed to estimate the exact effect on the productivity of cultures under environmental conditions. If we consider photoinhibition to be a physiological process that reduces the biomass production of Spirulina, the selection of strains belonging to each region with the capacity to tolerate and significantly improve the productivity of cultures under environmental conditions is a good alternative.
Conclusions
The results presented indicate that the incidental strong solar radiation on the culture system in a doubly curved tubular photobioreactor affects the morphology and productivity of A. platensis. Therefore, this solar radiation is not optimum for the culture system in photobioreactor in the open air, demonstrated in a low biomass productivity and chlorophyll content of 13.9 g/m2 and 0.00785 mg/L, respectively. Meanwhile, the temperature reached during the day proved to be adequate for the cultivation of A. platensis. More studies are required to discover and establish the optimum percentage of solar radiation to obtain good productivity of A. platensis in tubular photobioreactors.











 text in
text in