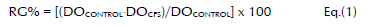Introduction
The growth of fungal species is one of the most frequent causes of deterioration of food, it entails great economic losses, and it can lead to public health problems because of the production of mycotoxins. Aspergillus ochraceus is an isolated mold of a wide range of food products. It grows in warm and tropical climates and it is a producer of ochratoxin A (OTA) (Soriano, 2007), a nephrotoxic mycotoxin that can also have hepatotox" ic, teratogenic and immunosuppresive properties, clas" sified by the IARC (International Agency for Research on Cancer) as a possible human carcinogen (Category 2B) (O'Callaghan et al. 2006). OTA can survive typical processes in the food industry and it is found in baked bread (Scudamore et al. 2003), beer (O'Callaghan et al. 2006) and coffee (Frank, 1999; Urbano et al. 2001; Taniwaki et al. 2003; Martins et al. 2003; Batista et al. 2003; Suarez-Quiroz et al. 2004). Some chemical fungicides are effective in controlling the growth of harmful molds, but their use is considered undesirable due to the possibility of hazardous residue remaining in the raw materials and/or foods that put the consumers' health at risk. Therefore, the search for new food preservation methods has steered toward more natural methods. For many years, consumers have demanded food preservation without chemical preservatives. The use of biological substances with antifungal properties could help the food industry to meet this requirement. A possible solution for these demands could be the use of naturally acidified products such as kefir; the antifungal activity and metabolites of which have been studied previously (Gamba et al. 2015; Miao et al. 2014; Garrote et al. 2000). Water kefir (WK) is a fermented, moderately acidic drink that is comprised of compounds including CO2 and ethanol (Garrote et al. 2001). For its preparation, sugar solutions are used that are fermented by a consortium of microorganisms, which are embedded in a polysaccharide matrix known as a WK grain (Waldherr et al. 2010) with an irregular shape and gelatinous aspect. The consortium of microorganisms is comprised of lactic acid bacteria (LAB), yeasts (Y) and acetic acid bacteria (AAB) (Waldherr et al. 2010; Diniz, et al. 2003), which live together in symbiosis. The fermentation and transfer of the WK grains may occur in non-sterile conditions, practically without risk of contamination. Said symbiotic relationship is very stable, because it does not permit extensive growth of foreign microorganisms (Moinas et al. 1980; Pidoux 1989). The stability of the grain seems to be based on the effect of the pH and the presence of inhibitory compounds or antagonistic substances that act against external, contaminating pathogens (Pidoux, 1989). The microorganisms embedded in the WK grain are capable of producing lactic acid, acetic acid and other biologically active compounds that could inhibit the growth of pathogenic and undesirable microorganisms (Silva et al. 2009) from the fermentation of any kind of sugar substrate. This research used panela, a sweetener obtained from the extraction and evaporation of sugarcane (Saccharum officinarum L) juice (Ministry of Social Protection, Colombia, 2006). It is a non-centrifugated, unrefined, natural and whole sugar, which is cheaper than sucrose (table sugar); and was used as a substrate for fermentation by the WK grains.
Once the fermentation of the substrate takes place, the carbohydrates are greatly reduced, which are mainly transformed into products with a low molecular weight that exhibit antimicrobial properties, with organic acids (OAs) being the most common (Gerez et al. 2009). Their action is based on the "weak acid theory", because they can achieve a decrease in the intracellular pH of microorganisms by the action of the non-disassociated portions of these acids because of their pKa (Bogaert & Naidu, 2000; León et al. 2012). This phenomenon is due to the hydrophobic properties that most of the OAs exhibit, which permits the free diffusion of their protonated form through the cellular membrane. This process takes place spontaneously because of the pH gradients and osmotic concentration that exist between the interior and exterior of the cell. The intracellular pH is higher than the extracellular pH, because the acids are freely permeable in the microorganisms' membrane, disassociating themselves in the cytoplasm and decreasing the pH through the release of protons (Bogaert & Naidu, 2000; León et al. 2012). These destabilize the intracellular pH, breaking its neutrality and the gradient of the cellular membrane (Salmond et al. 1984; León et al. 2012). The cell uses its energy in the form of ATP to expel the proton and maintain its viability, but it uses the energy necessary for its growth kinetics (Hunter & Segel, 1973; León et al. 2012). The main aim of the study was to investigate the capacity of cell-free supernatants (CFS) obtained from the fermentation of a panela solution (4.15% m/v) by WK grains (10% m/v) for inhibiting or delaying the in vitro growth of the toxigenic mold A. ochraceus AFUNL9 and FNSP. Other objectives of this research were as follows: i) comparison of the antifun-gal activity of the fermented products by WK grains with panela solutions artificially acidified with strong acids (3 M HCl) or with pure organic acids (lactic and acetic acid); ii) evaluation of the technological conditions of fermentation with KA grains; iii) biomass production; and iv) count of microorganisms (LAB, yeasts and acetic acid bacteria) at each assessed fermentation temperature (25 °C, 30 °C and 37 °C).
Materials and Methods
Cultivation of Water Kefir Grains
The WK grains were provided by the Microbiology Department of the School of Exact Sciences of Universidad Nacional de La Plata (La Plata, Argentina). They were continuously cultivated every 48 hours in recently prepared, previously boiled, aqueous panela solution, fermented at 25 °C.
Fermentation Process
The substrate used for the fermentation was prepared with powdered panela obtained from a local supermarket (Exito®, Medellin, Colombia), using tap water like in the traditional preparation in Colombia. All the solutions were heated until boiling for 1 minute.
A panela solution of 41.5 g/l was used, similar to the one traditionally used. When the solutions reached room temperature, they were inoculated with 10% m/v of WK grains. A control was used without the addition of WK grains. All the solutions were fermented at three different temperatures (25 °C, 30 °C and 37 °C).
Determining the Fermentation Kinetics and the Increase in Biomass
The pH values were measured every 30 minutes for 12 hours, and at 24, 28 and 32.5 hours of fermentation with a digital potentiometer (Thermo Electron Corp., USA). The biomass was determined with the drained weight of the grains using an analytical balance (Ohaus, USA) for ten days.
Identification and Ratio of the Groups of Microorganisms in Water Kefir
After fermenting the panela substrate for 32.5 hours at three different temperatures (25 °C, 30 °C and 37 °C), the viable microorganisms were numbered in the fermented drink prepared with 41.5 g/l of panela in three different culture media, De Man Rogosa and Sharpe (MRS) (Merck, Darmstadt, Germany), as a medium for the growth of LAB, oxytetracycline glucose yeast extract agar (OGYE) (Merck, Germany) for the yeasts and for the count of AAB, mannitol agar, prepared with D-mannitol (Sigma-Aldrich, St. Louis, MO, USA), yeast extract (Oxoid, Cambridge, England), peptone (Dibico, Mexico City, Mexico), and agar (Merck, Germany) (Hernández et al. 2008).
Obtaining the Cell-free Supernatants
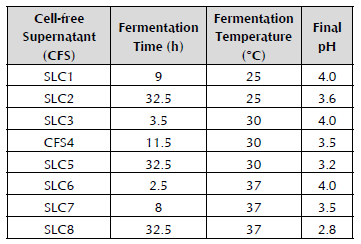
When the fermentation reached the final pH values (Table 1) established for the duration of the fermentation at the three temperatures (25 °C, 30 °C and 37 °C), the aqueous panela solutions fermented by the WK grains were filtered using a sieve with a mesh size of 1 mm2 in order to separate the grains from the fermented substrate. The fermented solutions obtained were centrifugated at 6,000 rpm for 15 minutes. (Hettich, Tuttlingen, Germany) in Falcon plastic tubes with a capacity of 15 ml to separate the bacteria and other particles after fermentation. The supernatants obtained were filtered through a sterile membrane with a pore size of 0.45 Mm (Advantec MFS, Dublin, CA, USA) using a sterile polysulfone filter holder (Advantec MFS) with air suction to create a vacuum. Some CFS (CFS3, CFS5 and CFS7) were subjected to boiling for five minutes. All the CFS (Table 1) were stored at -20 °C until their later use.
Analysis of the Cell-free Supernatants
The organic acids (OAs) (lactic and acetic) and the carbohydrates (sucrose, glucose and fructose) were analyzed by HPLC (Agilent Technologies Series 1200, Santa Clara, CA, USA) analysis using an ionic exchange column, Aminex HPX-87H (Bio-Rad, Hercules, USA). As a mobile phase, H2SO4 0.008 N was used at a flow of 0.6 ml/min and at a temperature of 35 °C. UV-visible spectroscopy was used for detecting the lactic acid and the refractive index for the acetic acid and the carbohydrates. The OAs and the carbohydrates were identified according to the retention time compared to the standard solutions of lactic acid (Merck, Germany) and acetic acid (Carlo-Erba, Italy), and of the carbohydrates: sucrose, glucose and fructose (Merck, Germany).
Preparation of the Inoculum of Aspergillus Ochraceus
Aspergillus ochraceus AFUNL9, isolated from cereal samples from the Microbiology Department of Universidad Nacional de la Plata (La Plata, Argentina) and A. ochraceus FNSP isolated from environmental samples from the Public Health Laboratory of Universidad de Antioquia (Medellin, Colombia) were cultivated in inclined test tubes with Sabouraud agar (Merck, Darmstadt, Germany) at 25 °C until they spored. The spore inoculum was prepared by washing the surface of the agar with sterile sodium laureth sulphate solution (Merck, Germany) at 0.01% (m/v) in glucose solution at 1% (m/v) (Carlo Erba, Milan, Italy). Ten milliliters of the solution were added to the culture, loosening the conidia by light scraping with a sterile spatula and serial dilutions were made. The number of conidia was determined by counting in a Neubauer chamber.
Biological Assay for the Reduction of Germination of Aspergillus ochraceus Conidia
The reduction of germination (RG) of the conidia of A. ochraceus AFUNL9 and A. ochraceus FNSP was carried out in sterile and disposable flat-bottom Petri dishes of microdilution (96 wells) (Brand, Wertheim, Germany) (Magnusson et al. 2003; Lavermicocca et al. 2000). The suspension (10 μl), which contained 104 conidia per ml, was added to 190 μl of each CFS in each well (Table 1). The assays were carried out eight times using the unfermented panela substrate as a control and which was sterilized by microfiltration like the other CFS. The RG of the conidia was determined after incubation for 48 hours in a humid chamber at 25 °C. For the DO readings, an automated ELISA RT-2100C microplate reader (Pioway, Nanjing, China) for assays was used at a wavelength of 492 nm (Rojas et al. 2004). The antifungal activity of the CFS was expressed as RG% measured at one DO492 compared to the control. The RG% was calculated using Equation 1:
An RG% of the conidia greater than 20% is considered to be positive. The reduction is low when it is under 40%, it is medium between 40% and 70%, and it is strong if it is more than 70% (Gerez et al. 2009).
Preparation of the Comparisons
Three panela solutions (41.5 g/l) were artificially acidified by adding lactic acid and acetic acid in the amounts reported in the analysis by HPLC. Said concentrations were as follows: 2.1542 and 0.1408 g/l (to simulate CFS7); 2.1011 and 0.3835 g/l (to simulate CFS5); and 0.6181 and 0.0375 g/l (to simulate CFS3) of lactic acid (Carlo-Erba, Italy) and acetic acid (Merck, Germany), respectively. In another group of experiments, the pH of three aqueous panela solutions (41.5 g/l) was adjusted to each one of the following pH values: 3.2; 3.5 and 4.0; using 3 M HCl (Merck, Germany) to simulate the pH values found in some treatments with a greater inhibitory effect. The pH of the supernatants was established at 25 °C with an Orion 710A+ analyzer (Thermo Electron Corp.). All of the artificially acidified panela solutions were centrifugated and microfiltered as described above.
Results and Discussion
PH Kinetics
The pH decreased as soon as the WK grains were added to the panela substrate and it continued to acidify in the fermentation time at the three assessed temperatures (25 °C, 30 °C and 37 °C). The control remained at a constant pH until presenting a slight decrease after 12.5 hours, possibly developed by yeasts that could contaminate it during the repeated pH readings when inserting the electrode in the drink. In general, it is observed (Figure 1A) that the acidification curve initially has a more pronounced slope in the first hours of fermentation where the pH decreases with greater speed. Later, the acidification slope is less pronounced and the pH decreases more slowly until presenting a trend to remain within a very close range of values, where the metabolic activity of the microorganisms and the acid production could possibly be inhibitory in the development of some microorganisms (Garrote, 1999). It was established that panela is an adequate substrate for the cultivation of the WK grains. It was also found that the pH decreased more quickly as the temperature increased.
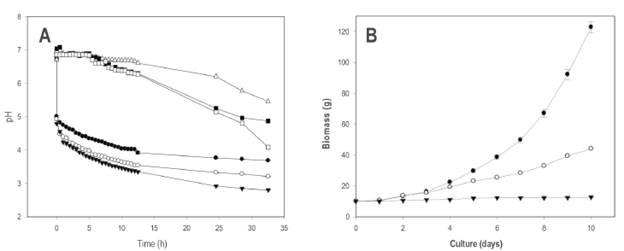
Figure 1 (A): Kinetics of pH for the panela substrate (4.15% panela and 10% WK grains). •: 25 °C, O: 30 °C, T: 37 °C, A: control 25 °C, ■: control 30 °C, and □: control 37 °C. (B): Increase in the biomass of the WK grains (10%) in panela solution (4.15%). •: 25 °C, O: 30 °C, T: 37 °C The error bars represent the standard deviation of a test carried out three times.
Some authors have reported a decrease in the pH in sugar substrates in order to be fermented by the consortium of microorganisms embedded in the WK grain. Silva et al. (2009) used 10 g/l of different unrefined sugars (demerara, brown sugar and molasses) as a fermentation substrate for WK (inoculum of 0.6%) in which the decrease in the pH over seven days was analyzed, adjusting the initial pH to 5 with 0.1 M HCl. These authors established the minimum pH of 3.3 for demerara sugar; of 3.4 for brown sugar; and of 3.3 for molasses, after 48 hours of incubation at 25 °C. This research found minimum pH levels of 3.7 in shorter periods of time (32.5 hours) at 25 °C, and in those which used panela as a substrate inoculated with 10% of the WK grain, which permits the conclusion that panela is an adequate medium for the cultivation of WK grains.
Using milk kefir, Garrote (1999) reported a decrease in the pH after 48 hours to a level of 4.5 when the milk was inoculated with 1% kefir. In this research, when using levels of 1% WK in the aqueous panela substrate, pH values of 4.3 and 4.2 (data not shown) were achieved at 32.5 hours of fermentation when said fermentation occurred at 25 °C, and even lower pH levels when fermentation was carried out at higher temperatures (30 °C and 37 °C). The greater capacity of WK to decrease pH compared to milk kefir may be due to the lack of proteins, calcium and phosphates in the panela substrate, which can act as buffer systems in a food matrix like milk (Edward & Herod, 1994).
The decrease in the pH is important because it is an indicator of acidification due to the conversion of carbohydrates to derivative byproducts of the fermentation process. These metabolic byproducts are important because their presence may be considered to be one of the main and effective means for the inhibition of pathogenic microorganisms, and therefore, they were quantified in this study.
Proportions of Viable Microorganisms
As observed in Table 2, it was found that the LAB had their maximum count at 25 °C and 30 °C; the yeasts were constant and the AAB had their greatest counts at 25 °C and 37 °C. In the fermentations carried out at 25 °C and 30 °C, it was observed that the LAB were the dominant group. This was not the case at the temperature of 37 °C, where there was an increase in the yeasts and a decrease in the AAB showing a different rearrangement. Previously, it has been confirmed that the proportion of yeast in WK grains represents 2 to 5% of the total flora (Hallé et al. 1994), but at this temperature, the proportion of microorganisms changed completely. This could entail consequences for the fermentation of the drink because it could increase its alcoholic content. Furthermore, the decrease in LAB could have a negative effect on the production of the polysaccharide that comprises the WK grain. The rearrangement regarding the microorganisms of the fermented drink by the WK grains at a temperature of 37 °C could also be attributed to the fact that greater metabolic activity developed at this temperature, at which there was a greater consumption of metabolizable components from the panela drink and subsequently, the depletion of nutrients consumed by the fermenting microorganisms. This, in turn, could translate into microbiological death and/ or the loss of microbial growth capacity. This could be firstly shown with a greater production of organic acids and a larger decrease in the pH, and secondly, in the total flora count of each one of the fermented products. At 25 °C, a total of 6.4 x 107 CFU per ml of total viable microorganisms (sum of all the microorganisms and yeasts) was quantified, while at 30 °C and at 37 °C, lower amounts of 4 x 107 and 4 x 106 CFU per ml were quantified, respectively. These results show that the fermentation temperature plays a crucial role in the symbiotic relationship of the microorganisms that comprise a WK grain. The decrease in the amount of microorganisms could play a very important role in maintaining the weight through successive peaks of the grain (Garrote, 1999). Some researchers have also reported the microorganism content of sugary drinks fermented with kefir grains. Texeira et al. (2010) fermented a solution of 5% brown sugar with kefir grains (approximately 10%), and after fermentation for 24 hours at 25 °C, they obtained a proportion of microorganisms in the fermented drink of 57.6% LAB, followed by the yeasts with 30.9% and the AAB with 11.5%. In this test, as reported in the present research, LAB were a dominant group, but a different proportion of microorganisms is observed, with lower LAB values (94%) obtained when the panela drink was fermented at the same temperature (25 °C). It is also observed that the amount of yeasts and AAB is greater than the amount found in this study in the fermented drink under identical conditions. This difference in the proportion of microorganisms could be due to the origin and conditions of fermentation of the grains, which could be a determining factor (Garrote, 1999). However, it is observed that there is a predominance of LAB compared to the other quantified microorganisms.
Table 2 Average count of the viable microorganisms in the WK. CFU: colony-forming units; LAB: Lactic acid bacteria; AAB: Acetic acid bacteria; SWI: Substrate with inoculum; SWNI: Substrate without inoculum; ND: Not detectable; and ±: Standard deviation. Tests conducted twice
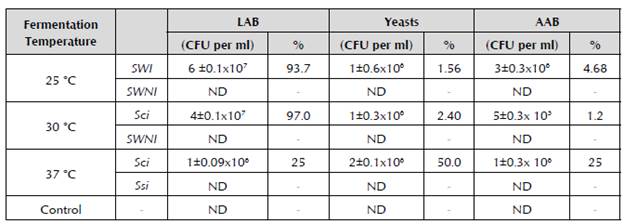
The results obtained at the fermentation temperatures of 25 °C and 30 °C are in line with those reported by Simova (2002) and Teixeira et al. (2010), who identified that LAB were the dominant and most numerous group of microorganisms in the milk kefir and water kefir grains, respectively. This indicates that the microorganisms that had a high count in the polysaccharide matrix passed to the drink, possibly because of their limited adhesion to the grain (Jianzhong et al. 2009).
Biomass Production
Figure 1B shows the growth rate of biomass expressed in grams. It was demonstrated that the incubation temperature had a significant effect (p < 0.05) after cultivation for ten days, obtaining the greatest increases (123 ± 3.7 g) at 25 °C. At 30 °C, an average final weight of 44 ± 0.4 g was obtained. At 37 °C, the lowest increase in biomass was obtained with an average final weight of 13 ± 0.3 g, the biomass increasing after ten days by only 3 g. Therefore, it can be stated that this was not an optimum temperature for the production of biomass from the grains. The WK grain is capable of fermenting substrates that are relatively poor in nitrogen for years (Hallé et al. 1994), as occurred with the panela when this parameter was analyzed in our laboratory (data not shown). Generally, the sugar available in the usual fermentation substrates is sucrose, but some microorganisms of the grain are incapable of metabolizing it or do so very slowly. Therefore, the yeasts benefit from the metabolism of the bacteria, because many of them have specific enzymes to divide this disaccharide; and in turn, the bacteria benefit from the vitamins and amino acids produced by the yeasts and released after their cellular lysis (Hallé et al. 1994). Diniz et al. (2003) carried out an incremental curve of the biomass of WK grains (5 g), cultivating them in a substrate comprised of molasses (50 g/l) at 25 °C, and obtaining around 41 g after ten days of incubation, which is an 88% increase in biomass (IB%). In the experiments conducted at 25 °C, a 92% IB was obtained, demonstrating the efficiency of our grains and of the panela substrate used for fermentation. These results contrast with milk kefir, in which it has been reported that the highest biomass production has been achieved at 37 °C instead of at 25 °C (Zajšej & Goršek, 2011).
The factors that affect the increase in the biomass of the WK grain have been studied very little, but when comparing them with the results previously obtained with milk kefir, it may be assumed that if a high ratio is maintained of lactic acid bacteria to yeast present in the grain matrix, a good increase in biomass is achieved (Guzel-Seydi et al. 2011).
These results corroborate that the production of the dextran polysaccharide compound that forms the WK grain is mediated by the LAB, this being one of the principal and most important tasks for its maintenance (Pidoux, 1989). The dextran gels have the ability to immobilize microorganisms and they can be produced by using economical substrates that contain sugars (Pidoux et al. 1992), such as panela.
Carbohydrate Metabolism
Sucrose, glucose and fructose were analyzed in the CFS through HPLC. The sucrose decreased compared to the control (unfermented substrate, UFS) in all the examined supernatants (Figure 2). The glucose and fructose did not significantly increase, which was possibly due to their use in the metabolic processes of the microorganisms of the WK grain, mainly to produce lactic acid. In CFS8 (pH: 2.8; obtained at the temperature: 37 °C), the greatest decrease in the initial concentration was obtained, approximately by 95%, followed by CFS5 (pH: 3.2; obtained at the temperature: 30 °C) (74%) and CFS2 (pH: 3.6; obtained at the temperature: 25 °C) (42%). These three supernatants had a higher fermentation time than the rest of the supernatants used (Table 1,). This greater contact time between the grains and the substrate may explain the greater hydrolysis of the sucrose and its later assimilation for the greater production of OAs and other products of the metabolism of the microorganisms present. Part of the sucrose can also be used for the formation of the exo-polysaccharide (EPS) through dextransucrase enzymes (Salminen et al. 2004).
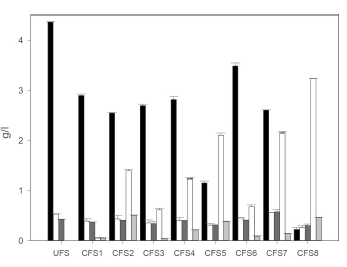
Figure 2 Quantification of carbohydrates and organic acids in the unfermented substrate (UFS) and the cell-free superna-tants (CFS). The error bars represent the standard deviation of a test carried out twice. Black bar: Sucrose; White bar: Glucose; Grey bar: Fructose; White zig-zag bar: Lactic acid; and Grey zig-zag bar: Acetic acid.
Analysis of Organic Acids
In order to determine whether the differences in fungal inhibition are due to the variations in the production of OAs, the CFS were analyzed by HPLC and the amount of lactic and acetic acids was determined. It is observed (Figure 2) that the amounts of lactic acid are greater than those of acetic acid in all of the supernatants obtained. This is possibly because the ho-mofermentative LAB are more numerous than the heterofermentative LAB and than the AAB (Teixeira et al. 2010); and because the amount of lactic acid produced increased with the incubation time and the temperature. None of these OAs could quantify in the control (UFS). Few reports have been found about the amount of OAs produced from the fermentation of sugar substrates by WK grains. In 2010, Teixeira et al. assessed the OAs (lactic and acetic) in a drink made from brown sugar (5% m/v) fermented with WK grains (11% m/v) for 24 hours at room temperature (25 °C), finding amounts of 19.42 mmol/l of lactic acid (approximately) and 23.31 mmol/l of acetic acid. In our fermentations also carried out at 25 °C, but during a period approximately eight hours longer, the concentration of lactic acid was a little lower at 15.5 mmol/l. Likewise, the concentration of acetic acid was almost two times lower at a value of 8.4 mmol/l. Unlike the results reported by Teixeira et al. (2010), in all our fermentations, the concentration of acetic acid was always less than that of lactic acid. This difference may be due to the amount and ratio of microorganisms that form the grain. In our case, at 25 °C, the amount of LAB, yeasts and AAB was 93.75%, 1.56% and 4.68%, respectively. Despite the fact that the LAB were the dominant group in the previously cited study with 57.65%, the proportions of the microorganisms were different with 30.86% yeasts and 11.48% AAB. These differences in the amounts of microorganisms, as well as the effect that the temperature might have had, could result in different proportions of OAs found in the drinks fermented by the WK grains.
The microorganisms that comprise the WK grain caused the hydrolysis of the sucrose in order to be metabolized together with the glucose and fructose that were converted into other substances, which could have important implications in the inhibition of pathogenic or food-contaminating microorganisms.
Biological Assay
After 48 hours of incubation at 25 °C, most of the supernatants presented an antifungal effect because they produced an RG% higher than 20% (Figure 3). CFS5 (pH: 3.2; obtained at the temperature: 30 °C) produced the highest RG% of 50% and 47% for A. ochraceus AFUNL9 and A. ochraceus FNSP, respectively. The only exception was presented by CFS6 (pH: 4.0; obtained at the temperature: 37 °C), which in both strains of A. ochraceus did not achieve germination. This inability could be related to the fact that this supernatant contained high rates of non-assimilated carbohydrates during the fermentation period, presenting the highest content of sucrose (3.5 g/l), only exceeded by the control (4.4 g/l), as well as high amounts of glucose and fructose (see Figure 2). These available carbohydrates could lead to a faster germination similar to the control. The RG% levels of the conidia of both strains of A. ochraceus at 48 hours of incubation had a statistically significant difference (p < 0.05). This is due to the fact that each supernatant obtained at different temperatures showed a unique aspect in the inhibition presented and where the concentrations of OAs could play an essential role in the inhibition. Each one of the supernatants contained different amounts of OAs produced and other metabolic products of fermentation. This particularity resulted in each supernatant producing clearly different results in the RG%, which demonstrates that time and temperature of fermentation were determining factors for the greater or lower inhibition shown in both strains of A. ochraceus. It would be expected that the supernatants with greater antifungal power would be those fermented at the highest temperature (37 °C), where there was the greatest amount of OAs (lactic and acetic), as can be appreciated in CFS8 (pH: 2.8; obtained at 37 °C) (Figure 2), which has the two characteristics (low pH and greater amount of OAs), but even then, it did stand out in the RG of the conidia of A. ochraceus. These results indicate that there may be compounds unrelated to the pH or the greater production of OAs in addition to the ones that were quantified (lactic and acetic), which could also reduce the germination of both strains of A. ochraceus at 48 hours of incubation.
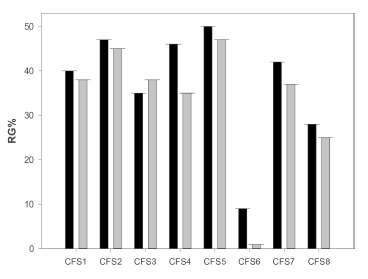
Figure 3 Reduction of germination percentage (RG%) of the conidia of A. ochraceus AFUNL9 and FNSP with the CFS (see Table 1). The error bars represent the standard deviation of a test carried out eight times. Black bar: A. ochraceus AFUNL9; and White bar: A. ochraceus FNSP.
Effect of the Organic Acids
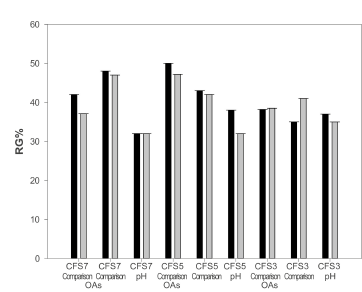
To assess whether the lactic and acetic OAs were to a large extent, the agents responsible for the inhibitory effects, comparisons were prepared of CFS7 (pH: 3.5; obtained at 37 °C), CFS5 (pH: 3.2; obtained at 30 °C), and CFS3 (pH: 4.0; obtained at 30 °C). The acids were added in the same concentrations to those found in the supernatants from the fermentations. The comparisons of the three supernatants acted similarly to the CFS on both strains of A. ochraceus, achieving the highest RG% with the comparison of CFS7 (48 ± 0.005% on A. ochraceus AFUNL9 and 47 ± 0.004% on A. ochraceus FNSP). Out of these three supernatants (and their comparisons), CFS3 contained the lowest amount of OAs and consequently was the one that produced the lowest RG% of both strains of A. ochraceus. We could assume that the OAs played an essential role in fungal inhibition, because the comparisons of the CFS reduced the germination of the conidia of A. ochraceus of both strains. As known, the non-disassociated form of the OAs is the main cause of their inhibitory capacity (Garrote et al. 2000). The capacity of these acids to reduce the pH of the medium and the lipophilic character of their protonated form (non-disassociated) facilitates their entry through the cellular membrane (Bogaert & Naidu, 2000).
Effect of the pH
To analyze the influence that the pH could have on the RG% of the conidia of A. ochraceus of both strains, the aqueous panela substrate was artificially acidified with 3 M HCl until reaching the three pH values of CFS7 (pH: 3.5), CFS5 (pH: 3.2) and CFS3 (pH: 4.0). The pH comparisons had an inhibitory effect, although they were significantly lower (p < 0.05) than those obtained with the supernatants fermented with WK grains (Figure 4). It could be assumed that, as well as the pH, other inhibitory substances influence the RG% of the fungus. In contrast with these results, Garrote et al. (2000) reported that they did not find that the pH had any inhibitory effect per se, when they acidified milk with HCl to inhibit Escherichia coli. However, when they added lactic acid and/or acetic acid, they found an inhibitory effect. In the results, the aqueous panela solutions artificially acidified with an inorganic acid (3 M HCl) had an inhibitory effect without the need for the presence of OAs (lactic acid and/or acetic acid) in the medium, although it was smaller. It could be assumed that there is an antimicrobial action because of the reduction of the extracellular pH. Additionally, although it is considered that the strong monoprotic acids, such as HCl, do not have antimicrobial activity like the OAs have (Bogaert & Naidu, 2000), it has been described that the effect of the pH largely depends on other factors, including the type of substrate, the incubation, the temperature, and the strain of mold (Gourama & Bullerman,1995).
Effect of Boiling the Cell-free Supernatants
CFS3, CFS5 and CFS7 were boiled for 5 minutes before carrying out the RG% test. CFS3 (pH: 3.5; obtained at 25 °C) maintained the same inhibitory action that it had without boiling; CFS5 (pH: 3.2; obtained at 30 °C) significantly decreased; and CFS7 (pH: 3.5 lost its antifungal activity (Figure 5). It is possible that the products formed during the fermentation time had a thermolabil nature, because they were probably destroyed, volatilized or denatured during the process. Powell et al. (2007) reported a bacteriocin produced by Lb. plantarum ST8KF isolated from milk kefir, which remained active after being subject to a temperature of 121 °C for 20 minutes. Therefore, it may be inferred that protein compounds like the bacteriocins can support elevated temperatures, maintaining their inhibitory activity without being denatured.
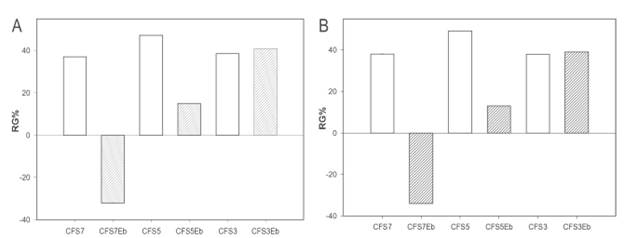
Figure 5 Comparison of the reduction of germination (RG) of (A) A. ochraceus AFUNL9 and (B) A. ochraceus FNSP after 48 hours of incubation at 25 "C between Cell-free Supernatants 7 (CFS7) (pH: 3.5), CFS5 (pH: 3.2) and CFS3 (pH: 4.G); and the CFS boiled (CFS b) for 5 minutes. The error bars represent the standard deviation of a test carried out eight times.
Conclusions
This study researched the fermentative activity of WK in a cheap and easily accessible and prepared substrate, panela. It was determined that the grain inoculated in this substrate and under different incubation conditions fermented until reaching minimum pH values of 2.8; maintained the count of the microbial groups of LAB, yeasts and AAB; and maintained its capacity to produce biomass. Additionally, the CFS obtained from the substrate fermented with WK grains demonstrated the capacity to inhibit the growth of A. ochraceus, making it a biopreservation alternative for food matrices.
Said antifungal activity was mainly attributed to the combination of the decrease in pH and the OAs produced during the fermentation of the substrate, especially, lactic acid and acetic acid. However, given the complex nature of the microbial community of the kefir grain, it will be necessary to identify the presence of other compounds with antifungal activity in the CFS.











 text in
text in