Introduction
For centuries, different cultures around the world have used fungi for nutritional and medicinal purposes, as well as in environmental processes thanks to their composition and to the metabolites they produce in both their natural environment and the laboratory (Zhao et al. 2010).
It has been found that many macromycete fungi have a potential anticarcinogenic effect, out of which, genera including Ganoderma, Lentinus, Pleurotus, Agaricus and Schizophyllum stand out (Chen et al. 2012; Benzie and Wachtel-Galor, 2011; Bishop et al. 2015; deVere et al. 2002). These genera are currently being used by different companies around the world, especially in East Asia, Australia and North America, for the production and sale of mushrooms and their extracts as a dietary supplement.
The Ganoderma lucidum species (reishi in Japanese and lingzhi in Chinese) has been widely studied around the world for its nutritional and medicinal properties. Historically, it has been known as the "elixir of life". This basidiomycete fungus is part of the fungi of white wood rod fungi, and it is recognized for its ligninolytic enzymes, as well as its medicinal properties, such as the prevention of obesity, maintaining intestinal health, reduction of high blood pressure, control of diabetes and the stimulation of probiotics (Bishop et al. 2015). The majority of research has been developed in East Asian countries (Korea, China and Japan) on topics related to the production, extraction and identification of the bioactive substances of Ganoderma. However, it is necessary to conduct more studies to increase knowledge about these fungi in countries with great biodiversity such as Colombia.
The Ganoderma polysaccharides have caught the attention of the scientific community because of the effects they have on the immune system, swelling and cancers with effects in vitro and in vivo, as demonstrated in different research (deVere et al. 2002; Daba and Ezeronye, 2003). Structurally, the fungal polysaccharides can be found in the form of homopolysaccharides, heteropolysaccharides and glycoproteins. In submerged cultures, both intracellular and extracellular polysaccharides (exopolysacharides) can be obtained. The intracellular polysaccharides (IPS) are found in the fungal cell wall and the exopolysaccharides (EPS) are extracellular polysaccharides released into the environment where the fungi are located and are mainly used as a protection mechanism (Donot et al. 2012). The medicinal properties of said polysaccharides depend not only on their chemical structure, molecular weight, and formation and configuration of glycosidic bonds, but also on the biological and physicochemical characteristics of the fermentation, including the culture media used, agitation and pH (Donot et al. 2012; Fraga et al. 2014). Although there is no consensus on the best conditions, the majority of authors suggests that greater EPS production occurs at low pH values and when using glucose as a substrate (Donot et al. 2012).
To date, more than 200 polysaccharides have been isolated from basidiocarps, spores, mycelia and liquid cultures of Ganoderma, the latter being the ones of preference for commercial exploitation, because it is difficult to find them growing naturally and basio-diocarp production takes several months. Additionally, the submerged cultures are easier to control and production is obtained in a short time. However, in the majority of cases, due to the production process and to the substrates used in the final products, it is very expensive and not very affordable for the general population (Wagner et al. 2004; Benzie and Wachtel-Galor, 2011). Therefore, it is important to assess different substrates for the production of polysaccharides with greater economic feasibility in order to make it a more affordable, good quality product. An alternative is to reduce the production costs by using agro-industrial waste that serves as a source of carbon and nutrients necessary for the development of the fungus and the generation of metabolites such as polysaccharides.
This research is based on the production of polysaccharides in a submerged culture on a laboratory scale and in a bioreactor from the isolation of Ganoderma sp. taken from the Andean region, using lignocellulosic waste, as a substrate, from the agricultural industry supplemented with glucose and lactose. Although some authors have not reached a consensus about the optimum source of carbon, it has been found that glucose facilitates the performance of EPS compared to other sugars (Wagner et al. 2004; Fraga et al. 2014). This fungus was selected for its great importance both industrially and medicinally, as well as its ligninolytic potential, corroborated in previous research conducted by the Biopolimer Research Group (Arboleda et al. 2008; Arboleda and Mejia, 2010).
Materials and Methods
Fungal Isolation
The strain of Ganoderma sp. used to conduct this work was collected from a rainforest in the municipality of Puerto Berrio (Antioquia, Colombia), thanks to a permit for the collection of fungi granted by the ANLA (Permit No. 17, 2012) to the Biopolimer Group, which has an interest in using the autochthonous microbiota. Preinoculations were carried out on the ligninolytic medium KIRK (Kirk, Croan et al. 1986) and potato dextrose agar (PDA). The incubation time was 30 ± 1 °C. The strains were preserved at 4 °C in the culture medium of malt extract agar (MEA). Staining was carried out with lactophenol blue, Congo red and potassium hydroxide (KOH) to identify the structures of the fungus and to monitor the culture in an Erlenmeyer flask as well as in a bioreactor.
Evaluation of Substrates for the Production of Biomass and Polysaccharides
A mix of organic waste containing carbon, nitrogen and appropriate growth factors for the development of the fungus was used as a base substrate. For the purposes of the research, un¡form¡ty ¡n the compos¡t¡on of the med¡um ¡s ensured and the substrate w¡ll be called B¡o, because ¡t was an undef¡ned culture med¡um that was patented by the B¡opol¡mer Research Group at the Colomb¡an Super¡ntendence of Industry and Commerce (Registration No. 11-160567, 2014), and ¡t was l¡censed to the sp¡n-off BIOINNCO S.A.S.; a company created from the results of the research by the Biopolimer Group.
Glucose and lactose were assessed ¡ndependently as ¡nducers of polysacchar¡des at three levels (1%, 10% and 20% p/v). Upon complet¡on of the fermentat¡on (10 days) the b¡omass produced ¡n grams per l¡ter and the concentration of EPS and IPS in grams per liter were measured as response var¡ables. The data were treated through the statistical analyses of ANOVA and Fisher's least s¡gn¡f¡cant d¡fference (LSD) test, us¡ng the software STATGRAPHICS Centurion XVI Version 16.1.02.®
Fermentation in Submerged Culture in a Laboratory
Batch fermentat¡ons were carr¡ed out ¡n 250-ml w¡de-mouth Erlenmeyer flasks w¡th cotton-gauze stops to fac¡l¡tate the transfer of gases, because oxygen ¡s essential for the growth of the fungus. The fermentations were carr¡ed out ¡n an orb¡tal shaker for 25 flasks w¡th temperature control. Each Erlenmeyer flask was taken as an exper¡mental un¡t. The tests were carr¡ed out separately and each one was repeated three t¡mes to obta¡n the standard dev¡at¡on.
In all cases, three d¡scs w¡th a d¡ameter of 5 mm were used as an inoculum from the trophophase of the prenoculum in Kirk medium with eight days of incubation. They were added in aseptic conditions to 250-ml Erlen-meyer flasks, with a fermentation volume of 40 ml, and Bio substrate was added at 3% p/v, as well as the corresponding sugars for each test. They all were adjusted to an initial pH of 4.0 ± 0.1, and they were incubated with orbital shaking at 150 rpm and 30 ± 1 °C for ten days.
The dry we¡ght of b¡omass was obta¡ned by f¡ltrat¡on. Firstly, a 100 pm-sieve was used, the biomass was washed three t¡mes w¡th de¡on¡zed water, and ¡t was f¡ltered through Whatman Grade 1 f¡lter paper to later be dr¡ed at 70 °C unt¡l reach¡ng constant we¡ght.
F¡gure 1 shows the process of extract¡on of polysacchar¡des. The polysacchar¡des were quant¡f¡ed by the phenol-sulphur¡c ac¡d method w¡th some mod¡f¡cat¡ons that are listed below (Masuko et al. 2005; DuBois et al. 1956): A mother solution of glucose was prepared at a concentrat¡on of 400 ppm and four d¡lut¡ons were made between 10 and 80 ppm, w¡th wh¡ch the cal¡bration curve was drawn. For the readings, 400 pl of glucose solution were taken and 200 pl of phenol were added, which were immediately added to 1000 pl l¡qu¡d of sulphur¡c ac¡d concentrate, the react¡on of wh¡ch generates a yellow¡sh sta¡n¡ng. The absorbency of the ¡nd¡cated wavelength for hexoses was read us¡ng a spectrophotometer, and ¡t amounted to 490 nm (UV-Vis Varian CARY 50 Bio) (DuBois et al. 1956).
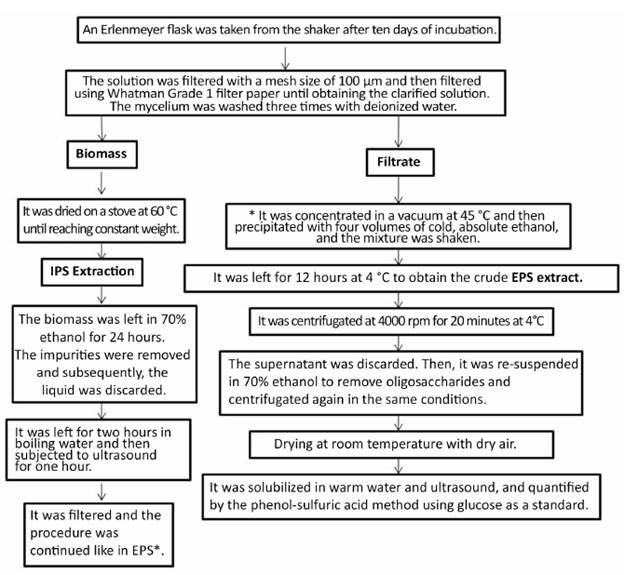
Figure 1 Procedure used for the extraction of polysaccharides from submerged culture of Ganoderma sp. It was conducted by modifying procedures of several authors (Donot et al. 2012; Nie et al. 2013; Ruthes et al. 2015).
Fermentation in Submerged Culture in a Five-Liter Bioreactor
A seven-l¡ter BIOSTAT® Aplus brand b¡oreactor was used. The cond¡t¡ons were as follows: 30 ± 1 °C, 300 rpm, 5 l and 1 vvm. The pre¡noculum used was from Ganoderma sp. in Bio medium + glucose at 10% w¡th f¡ve days of ¡ncubat¡on, wh¡ch represented 10% of the work¡ng volume of the b¡oreactor. The b¡omass and polysacchar¡des were determ¡ned ¡n the same way as w¡th the product obta¡ned ¡n Erlenmeyer flasks w¡th some mod¡f¡cat¡ons, wh¡ch are descr¡bed below. After the extract¡on process shown ¡n F¡gure 1, a sample of crude extract of polysaccharide was taken, and dialysis was carr¡ed out for three days w¡th de¡on¡zed water at 4 °C. Then, the samples were lyoph¡l¡zed, obtain¡ng four extracts named as follows: GEPSs: Soluble extracellular polysacchar¡de of Ganoderma, GEPS¡: Insoluble extracellular polysacchar¡de of Ganoderma, GIPSs: Soluble ¡ntracellular polysacchar¡de of Ganoderma, GIPS¡: Insoluble ¡ntracellular polysacchar¡de of Ganoderma.
For the UV tests, 20 mg/ml of each one of the extracts was taken; GEPSs and GIPSs were diluted in deion¡zed water, and GEPS¡ and GIPS¡ were d¡ssolved ¡n 1 M NaOH. Its fract¡ons between the wavelengths 200 and 400 nm were scanned to ¡dent¡fy prote¡ns (280 nm) and nucleic acids (260 nm) (Zhao et al. 2015). Then, the percentage of α-glucans and p-glucans present ¡n the extracts was found through the enzyme k¡t K-YBGL ® (Megazyme). Subsequently, the ¡nfrared ¡nstrumental techn¡que was used to ¡dent¡fy character¡stic bonds of polysaccharides in the ranges. The sample was prepared w¡th KBr ¡n spectroscop¡c grade powder to form a tablet of 1 mm, and later, the ¡nfrared measurements were made between the wavelengths of 1,000-4,000 waves per cent¡meter (Ramos, 1990; Zhao et al. 2015).
Biological Activity Assay
B¡oact¡v¡ty assays were conducted (Zhao et al. 2015; N¡e et al. 2013) on the extracts to determ¡ne the¡r ant¡-tumor capacity in cultures of carcinogenic cellular lines with the support and expertise of the GIM Research Group of Universidad de Antioquia. Mouse sarcoma J774 cell l¡nes were used to assess the ant¡tumor effect and VERO cell lines to assess the cytotoxicity in human cells, kindly donated by Laboratory 6, of the Cancer Research Center of Universidad de Salamanca, Spain. These were grown in RPMI 1640 (Gibco®) medium and supplemented with 10% of FBS (Fetal Bovine Serum), 1,000 U/ml of penicillin and 100 pg/ml of streptomyc¡n. For the treatment w¡th the extracts, the cells were cultivated at 1,500 cells/dish in 96-well Petri dishes at 37 °C and 5% of CO2. To calculate the inhibitory concentration 50 (IC50), a colorimetric assay (MTT) was conducted, which measures the cellular proliferation. The cells were resuspended in RPMI medium with 10% SFB and cultivated in 96-well Petri dishes (100 pl of final volume per well) at 37 °C and 5% of CO2 for 72 hours in the presence of different concentrations of each one of the polysaccharides (3.12, 6.25, 12.5, 25, 50 and 100 pg/ml) (Zhao et al. 2015; Nie et al. 2013). Each one of the concentrations was made three times.
Results and Discussion
Strain of Ganoderma sp.
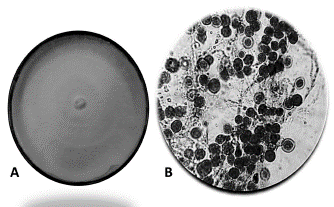
Figure 2 presents the Ganoderma sp. culture in Kirk medium and the microscopic observations in lacto-phenol blue, where the septate hyphae and chlamydo-spores characteristic of the fungus could be identified.
Evaluation of Substrates for the Production of Polysaccharides and Biomass of Ganoderma sp.
It has been found that the substrate affects the production of fungal metabolites, including polysaccharides, because some researches have demonstrated that part of the chemical composition of EPS and IPS to a certain extent depends on the culture medium in which the microorganism is incubated, mainly on the source of carbon (Donot et al. 2012; Kagimura et al. 2015).
Evaluation of Substrates for the Production of Biomass of Ganoderma sp.
Substrates used: Bio 3%; Bio 3% + Lactose (Lac: 1% 10% and 20%); Bio 3% + Glucose (Glu: 1%, 10% and 20%); and Bio 3% + Glucose + Lactose (Bio 3% + 10% Gluc + 10% Lac). The culture media that generated the greatest production of biomass were Bio 3% + Glucose 20% and Bio 3% + Lactose 10%.
The error bars correspond to the standard deviation of the experiments carried out three times for each variable.
The letters on the bars represent the significant differences between groups. The same letters indicate that there are no significant differences and different letters indicate that there are significant differences (p < 0.05).
The Bio substrate was used in this work with the aim to take advantage of biodegradable waste that can be used by the fungal enzymes, facilitating the production of metabolites, reducing production costs, obtaining good returns, and as added value, contributing to environmental conservation.
At the start of fermentation, the Bio culture medium presented partial solubility in water, showing changes during the fermentation process, observing transformation and solubility of the medium over time. This indicated that the fungus was consuming the substrate, compared to the control without inoculation. Figure 3 shows that said substrate in itself is not sufficient to achieve high concentrations of biomass in less time, obtaining an average of 12 g/l after 10 days of fermentation (Figure 3, Bio 3%). Other research, such as that by Fang and Zhong (2002), has used conventional sources of carbon, such as glucose at 20, 35, 50 and 65 g/l, finding that at the concentration of 35 g/l (approximately 3.5% p/v), 14.1 g/l of biomass of G. lucidum had been produced after eight days of fermentation. Furthermore, Tang and Zhong (2002) assessed the production of biomass with initial lactose at 20, 35, 50 and 65 g/l, obtaining a maximum of 14.39 g/l of biomass of G. lucidum at 65 g/l of lactose (approximately 6.5% p/v) after 14 days.
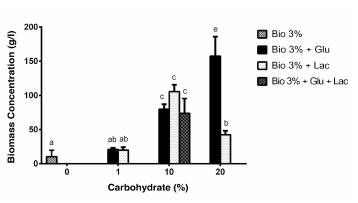
Figure 3 Effect of the concentration of different substrates on the production of biomass from Ganoderma sp.
In this research with the Bio medium at 3% not supplemented with glucose or lactose, 12 g/l was produced, and with glucose or lactose at 1%, 20.97 and 20.02 g/l were produced, respectively, after 10 days. This suggests that it is possible to use alternative, economic and environmentally-friendly substrates for the production of fungi of biotechnological importance with similar or better results than the conventional media.
In Figure 3 and in the analysis of the comparison of means through Fisher's LSD method, it can be concluded that the concentration of the biomass is similar to the one when using glucose or lactose at 1% and at 10% (independently as well as combined), with the 10% concentration being the one that facilitated the production of biomass the most. Other researchers, such as Papinutti (2010), also demonstrate that there are no significant differences in the development of biomass when glucose or lactose is used as the only source of carbon at an initial concentration of 1% p/v.
Additionally, it was observed that the substrate has a highly significant effect on the growth of the fungus. In all the percentages from where glucose or lactose was added, there was an increase in the concentration of the biomass, which was directly proportional in the case of glucose. When using lactose, an increase of up to 10% was observed, and from then on, it seemed to have inhibition by substrate. In other research, it has been demonstrated that high concentrations of some nutrients inhibit the growth of fungi in fermentation processes (Fang and Zhong, 2002; Chen et al. 2008; Papinutti, 2010).
Although the highest concentration of biomass is obtained at 20% glucose (200 g/l), it is a very high amount of substrate for a fermentation process that is intended to be used on an industrial scale, where the aim is to reduce production costs per batch, use alternative substrates and obtain good returns (cost-benefit). Additionally, 20% lactose is not very soluble in water and it hindered the growth of the fungus.
Evaluation of Substrates for the Production of IPS of Ganoderma sp.
Substrates used: Bio; Bio + Lactose (Lac: 1, 10 and 20%); Bio + Glucose (Glu: 1, 10 and 20%); and Bio + Glucose + Lactose (Bio + 10% Glu + 10% Lac). The culture medium that generated the greatest production of EPS was Bio + Lac 20%.
The error bars correspond to the standard deviation of the experiments carried out three times for each variable.
The letters on the bars represent the significant differences between groups. The same letters indicate that there are no significant differences and different letters indicate that there are significant differences (p < 0.05).
In Figure 4, it can be observed that the production of EPS is dose-dependent, having a greater concentration after the addition of glucose or lactose at 20%.
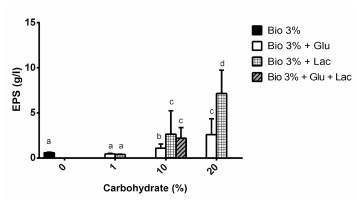
Figure 4 Effect of the concentration of different substrates on the production of EPS from Ganoderma sp.
Reviewing the results of other authors, it was found that Fang and Zhong (2002) had produced 0.43 ± 0.05 g/l of EPS of G. lucidum after six days of fermentation using glucose at 20 g/l (2% p/v) as a source of carbon; while Tang and Zhong (2002) had produced 0.32 ± 0.04 g/l of EPS of G. lucidum after 12 days of fermentation using lactose at 20 g/l (2% p/v). In this research with the Bio medium at 3% supplemented with glucose or lactose at 1%, 0.47 ± 0.06 g/l and 0.40 ± 0.04 g/l of EPS had been produced, respectively, after ten days of fermentation.
Statistically, significant differences were not observed when using lactose at 10% or glucose in combination with lactose at 10%. When using lactose or glucose at 10% and lactose at 20%, significant differences were observed in the production of EPS, which were greater in the case of lactose. This demonstrates that the production of EPS is dose-dependent with respect to the addition of these sugars, just has been demonstrated by other authors (Fang and Zhong, 2002; Tang and Zhong, 2002; Lee et al. 2007). Taking this into account, in order to economically and viably produce EPS, it is suggested to use the Bio substrate with lactose at 10% (2.65 g/l).
Evaluation of Substrates for the Production of IPS of Ganoderma sp.
Substrates used: Bio + Lactose (Lac: 1, 10 and 20%); Bio + Glucose (Glu: 1, 10 and 20%); and Bio + Glucose + Lactose (Bio + 10% Glu + 10% Lac). The culture medium that generated the greatest production of IPS was Bio + Glu 10% + Lac 10%.
The error bars correspond to the standard deviation of the experiments carried out three times for each variable.
The letters on the bars represent the significant differences between groups. The same letters indicate that there are no significant differences and different letters indicate that there are significant differences (p < 0.05).
Figure 5 shows that there are no significant differences when using the majority of the substrates for the production of IPS, except when using lactose at 20% and the combination of lactose and glucose at 10%, as observed in the different letters over the bars.
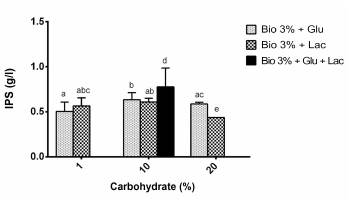
Figure 5 Effect of the concentration of different substrates on the production of IPS from Ganoderma sp.
When they used glucose at 20 g/l (2% p/v) as a source of carbon, Fan and Zhong (2002) had produced 0.55 ± 0.028 g/l of IPS of G. lucidum after six days, while Tang and Zhong (2002) produced 0.92 ± 0.00 g/l of IPS of G. lucidum using lactose as a source of carbon. This research had produced 0.5 ± 0.1 g/l and 0.56 ± 0.09 g/l of IPS using the Bio medium with glucose and lactose at 1%, respectively, after ten days of fermentation. In the case of glucose, the same result was obtained as the authors mentioned above, using agro-industrial waste as a substrate and at half of the glucose concentration that they used.
Using the Bio medium with 1, 10 and 20% p/v of glucose, the productivity of IPS related to the biomass was 25.7 ± 2.8; 8.72 ± 1.8; and 3.73 ± 0.76 mg/100 mg of biomass, respectively. Using the Bio medium with 1, 10 and 20% p/v of lactose, 33.95 ± 6.3; 9.06 ± 2.2; and 11.05 ± 2.3 mg/100 mg of biomass were obtained, respectively. This indicates that the production of IPS is inversely proportional to the concentration of carbohydrate added, similar to that found by Lee et al. (2007) in the production of IPS of G. applanatum.
The stimulatory effect of carbohydrates on the production of polysaccharides has already been observed in previous research (Fang and Zhong, 2002; Tang and Zhong, 2002; Lee et al. 2007; and Papinutti, 2010). From the results obtained, it can be concluded that the production of biomass and EPS in the majority of the cases is dose-dependent with respect to the supplemented carbohydrate, and in the case of IPS, no large differences were observed between the treatments. The greatest production of IPS was observed when combining the Bio medium with lactose and glucose at 10% (0.78 ± 0.1 g/l). This means that the production of biomass and production of polysaccharides are not necessarily directly related. Therefore, for the purposes of simultaneous production of biomass, EPS and IPS to obtain the best returns economically, it was decided to continue with the assays in the bioreactor with the Bio medium supplemented with lactose at 10%.
Production of Biomass and Polysaccharides in a Five-Liter Bioreactor
For the five-liter bioreactor, it was decided to work with lactose at 10% as a substrate to promote the production of biomass, as well as the production of poly-saccharides. Variables were not modified because production is carried out to obtain large amounts of biomass and polysaccharides for the subsequent analyses of identification. The culture was monitored in the microscope every two days, observing good formation and size of pellets, which is very positive when scaling up fermentation (Figure 6). Upon completion of the fermentation (five days), a biomass of 153 g/l was obtained. The fermentation time was less than the time at the Erlenmeyer flask scale, because a large amount of polysaccharides was generated in subsequent days, changing the rheological properties of the medium, hindering agitation and decreasing the transfer of oxygen (data not shown).
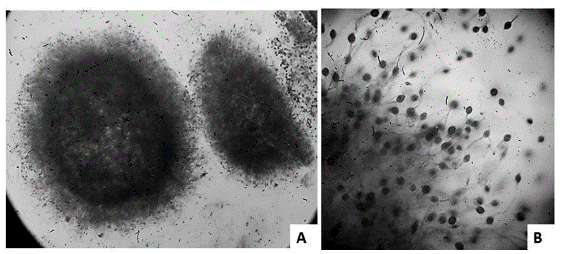
Figure 6 Monitoring of the pellets of Ganoderma sp. in submerged culture. Micrographs at 40X (A) and 400X (B) with lactophenol blue.
In Figure 7, the amount of polysaccharide produced by bioreactor volume is observed for Ganoderma sp. The greatest amount of carbohydrates obtained with the Bio medium supplemented with lactose occurs in the GIPSi extract and the lowest amount in the GIPSs extract. The total sum of polysaccharides produced in the bioreactor was 26.9 g/l. Due to the large amount of biomass produced, a greater concentration of insoluble IPS was obtained, because structurally, the fungal cell wall has insoluble polysaccharides such as chitin and glucans (Chan et al. 2009; Donot et al. 2012). From the EPS released in the medium, a greater proportion of soluble than insoluble polysaccharides was obtained, probably because the fungus was in an aqueous medium that facilitated the release of soluble substances.
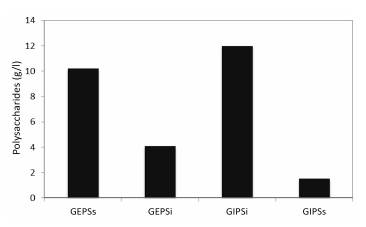
Figure 7 Production of EPS and IPS in a bioreactor. Ganoderma sp.: GEPSs: Soluble EPS; GEPSi: Insoluble EPS; GIPSi: Insoluble IPS; and GIPSs: Soluble IPS.
The α-glucans and β-glucans were quantified to determine the differences between the concentration of sugars obtained by the phenol-sulphuric acid method and the amount of α -glucans and β -glucans present in the extracts according to the most specific enzymatic method. As shown in Table 1, the presence of α -glucans and β -glucans in the extracts of Ganoderma sp. is confirmed. The GIPSi extract presented the greatest percentage of total glucans. In all the samples, it was found that out of the total polysaccharides of the different fractions, in general, 40 to 60% is comprised of glucans. This is highly satisfactory because these present good biological activity (Zhao et al. 2015; Nie et al. 2013; Ruthes et al. 2015).
Table 1 Weight/weight percentage of α -and β -glucans in the fungal extracts. The percentage is obtained based on the dry weight of the crude extract.
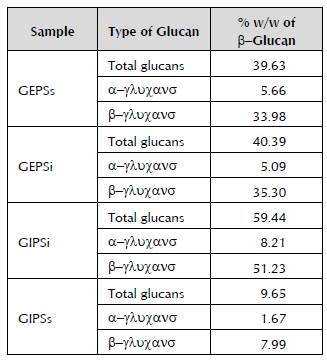
It was found that more than 80% of the total glucans are β -glucans, which is in line with the research on these fungi, because the β -glucans form part of their cell wall (Ruthes et al. 2015). This is highly satisfactory because the β -glucans have been the most studied, isolated and used glucans because of their bioactive properties.
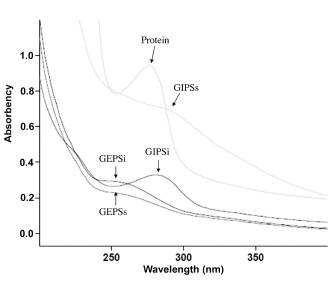
Analysis by Ultraviolet
Figure 8 shows the ultraviolet spectra of each one of the extracts at a wavelength between 200-400 nm. The extracts were compared against a protein standards, all at a concentration of 2 mg/ml. It can be observed that there are no characteristic peaks of nucleic acids (260 nm), and the GIPSi extract presented a peak at 280 nm. This allows the presence of proteins that can be joined to polysaccharides to be inferred, although in a small proportion due to their low absorption in the spectrum. To determine polysaccharides by the phenol-sulphuric acid method, the purity of the samples must be taken into account, because the method is sensitive to high concentrations of proteins. To purify them, it is common to use the Sevag method (Staub, 1965), which is very time consuming, reactive and highly laborious. As no high concentrations of protein were observed and the essential interest of this work is the bioprospection of endogenous fungi of Antioquia and the identification of metabolites of pharmaceutical interest, it was decided not to purify the extract in order to discover its characteristics and not to exclude possibly bioactive metabolites that are lost in the purification processes.
Infrared Analysis
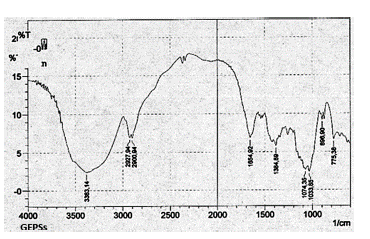
The determination of the anomeric configuration of the monosaccharides was analyzed through infrared (IR) spectroscopy. All of the extracts were analyzed and it was observed that they presented a similar pattern, therefore only the spectrum of GEPSs is presented.
The spectrum obtained for the GEPSs extract presented in Figure 9 was the expected spectrum for a β-glucan, showing an absorption band of around 890 waves per centimeter, which is characteristic of the β -glycosidic bonds. Furthermore, the spectrum lacks bands of 850 and 930 waves per centimeter, which are bands normally attributed to the α-glycosidic bonds (Limberger-Bayer, de Francisco et al. 2014).
In this spectrum, bands of 1,650 waves per centimeter are also observed, which are characteristic of the CO-NH bonds, related to peptides or amino sugars (Zechner-Krpan et al. 2010).
Biological Activity
Many studies have focused on the production and bio-activity of polysaccharides soluble in water, because they are easier to handle, quantify and characterize. In contrast, the activity of polysaccharides insoluble in water has been poorly studied (Papinutti, 2010). This is why this study evaluates the activity of soluble and insoluble polysaccharides, expanding the knowledge of the latter a little further.
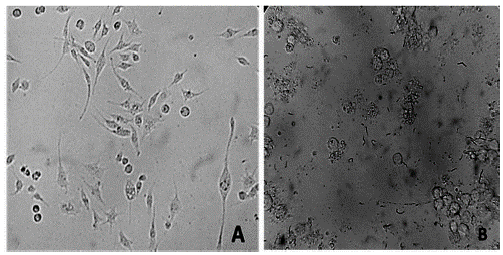
In the assays conducted on VERO cells, no growth inhibition was observed at the highest concentration used. Therefore, this indicates that the polysaccharides do not have cytotoxic activity in human cells at the evaluated concentrations (IC50 > 100 μg/ml) (data not shown) (OPS 2013). In reticular cell sarcoma of mice (J774), there was a morphological change and growth inhibition in cells at a concentration of 200 μg/ml (Figure 10).
Figure 10. A. J774 mouse sarcoma before treatment with the extracts. B. Sarcoma J774 after treatment with the extracts at 100 and 200 μg/ml.
The behavior was similar in all of the extracts.
In this research, a dose-dependent effect was observed similar to that reported by other authors regarding the antitumor activity of the in vitro polysaccharides (Chen et al. 2008).
The mechanisms of action of the polysaccharides on the carcinogenic cell lines is not completely explained (Zhao et al. 2015).
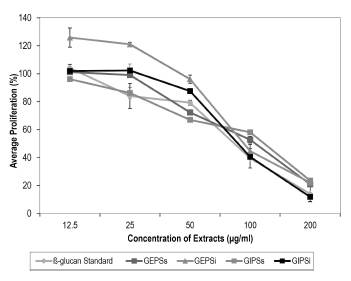
It can be observed that the effect is dose-dependent and that all the extracts have a similar activity to the standard p-glucan.
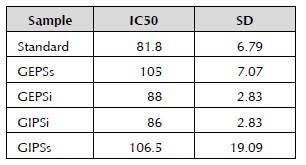
In Figure 11, an effect is observed of the Ganoderma sp. extracts on the percentage of sarcoma J774 cell proliferation. The control of the cells without treatment is 100%. Almost all the extracts present a similar behavior, including the β-glucan standard (Chromadex®), of which the sales promise is to have bioactivity. The effect is dose-dependent, where at low concentrations, there is no effect on the J774 cells, and an inhibitory effect on proliferation is observed at concentrations between 100 and 200 μg/ml. The extract that had the greatest effect on the cells and presented a similar behavior to the standard (IC50 = 81.8) was GIPSi (IC50 = 86) (Table 2). This extract presented a similar spectrum in IR to GEPSs (Figure 9), which indicates that it has bonds specific of the fungal polysaccharides. Additionally, it was the extract with the greatest concentration of sugars by bioreactor volume, as well as showing presence of proteins in the UV spectrum, and it was the one that obtained the highest percentage of glucans (59%) (Table 1, Table 2, and Figure 8). This indicates the importance of continuing to evaluate this extract, because in the assays conducted to date, it has all the characteristics of a bioactive polysaccharide. It is also necessary to conduct subsequent assays with other cell lines that evaluate their direct antitumor capacity or whether the effect is immunomodulatory, as has been demonstrated in other research (Feng et al. 2010).
Conclusions
Commercial strains are commonly used in research for the production of metabolites of industrial and pharmaceutical importance. In this work, high yields of biomass and polysaccharides were obtained in an Erlenmeyer flask and in a five-liter bioreactor from a Colombian strain of Ganoderma sp., isolated in the Andean region.
With the Bio substrate used, greater amounts of biomass and polysaccharides were obtained than those reported in research that used conventional substrates. Additionally, when using carbohydrate supplements such as glucose and lactose, the simultaneous production of biomass and polysaccharides improved, obtaining large amounts. Out of the evaluated media, the Bio medium supplemented with lactose at 10% was selected as the best substrate.
This work shows that it is possible to use agro-industrial waste in biotechnology processes, such as low-cost substrates, which contribute to the use of clean strategies that allow environmental conservation, and with which greater yields can be obtained.
The phenol-sulphuric acid, ultraviolet, percentage of glucans and IR assays allowed the presence of polysaccharides and some proteins to be detected in the extracts. This suggests that the endogenous fungi of our region also have important medicinal metabolites and characteristics like those reported in Eastern countries. It was found that more than 80% of the total glucans obtained is comprised of p-glucans, which have been widely studied for being bioactive molecules.
Additionally, it was demonstrated that the polysaccharide extracts at less than 200 μg/ml did not present toxicity in the VERO cell lines and have an antiproliferative effect on J774 sarcoma cells, especially with the GIPSi extract, which had a similar effect to the one generated by commercial standards of β-glucans.
It is necessary to conduct subsequent assays that allow to characterize the isolated polysaccharides and determine their biological activity, especially when the aim is to take advantage of the Colombian biodiversity.











 texto em
texto em