Introduction
Bananas (Musa spp.) are an important source of food for a large part of the global population, and more than five million hectares of banana are farmed around the world with an annual production of 106,714,204.76 tons (FAO, 2015).
Around 150,000 tons of banana are produced annually in Cuba (FAO, 2015) and the crop facilitates the stability of food products on the market, because of the capacity to harvest bananas every month of the year, deep-rooted consumption habits and the diversity of its uses (Rodríguez, 2000).
However, since November 1990, the appearance of a disease known as "black sigatoka" caused by the fungus Mycosphaerella fijiensis Morelet has been detected in Musa crops. This has severely affected the areas for farming plantains and bananas susceptible to this disease, which have been progressively replaced by other cultivars (Sánchez et al. 2002). A possible solution for this problem has been the introduction of banana and plantain hybrids from the Honduras Foundation for Agricultural Research (FHIA, for the Spanish original) that are more resistant or tolerant to the disease. These include the plantain cultivar 'FHIA - 25' (AAB), which has an excellent yield and is highly resistant to "black sigatoka". However, its fruit has the disadvantage of low sugar content, having the inherent limitations of the classic improvement because the main commercial cultivars are sterile, do not produce seeds and most of them are parthenocarpic (Stover and Sim-monds, 1987). In light of this situation, it is a priority to have a plant cell regeneration method, such as somatic embryogenesis, which complements biotechnological techniques of genetic transformation to improve the quality of the product (Kamle et al. 2011).
Somatic embryogenesis (SE) is a morphogenesis pathway in which an embryo is formed from a somatic cell or groups of them. The embryo is not the result of the fusion of gametes (Merkle et al. 1995), and it is based on the theory that all plant cells have the capacity to form complete plants (Steward et al. 1958).
Somatic embryogenesis is an auxiliary tool for bio-technological techniques to improve the genetics of bananas (Perea, 2001; Escalant and Jain, 2004), as well as permitting greater yields in a shorter period of time at lower costs, which makes this method potentially more efficient than regeneration via organogenesis (Ibaraki and Kurata, 2001).
Taking into consideration the aforementioned, the research was developed with the aim to establish a methodology for the regeneration of plants via somatic embryogenesis from the initial explant from apexes of axillary shoots.
Materials and Methods
The research was conducted at the Research Institute of Tropical Root and Tuber Crops (INIVIT, for the Spanish original) located in Santo Domingo, Villa Clara, Cuba. It was conducted in the period from January 2012 to February 2013.
The 'FHIA - 25' cultivar from the Germplasm Bank of the INIVIT was used, because of the institute's interest in the Plantain Genetic Improvement Program in Cuba. The explants to establish the in vitro aseptic cultures were apexes obtained from previously selected flowering plants in good phytosanitary condition. "Sword" offsets with a height between 25 and 30 cm were selected and put under semi-controlled conditions in a lath house covered with plastic mesh (Saran), which achieves a 70% reduction in the light intensity during this stage. They were watered by micro irrigation through a Microjet system at a frequency of six irrigations per day, each lasting two minutes. A relative humidity of 85-90% was ensured. After 45 days, the explants were disinfected and established in vitro in the culture medium proposed by López (1999). The materials were incubated for 15 days in a culture chamber at a temperature of 27 ± 2.0 °C and artificial lighting, with a system of 16 hours of light at a photosynthetic photon flux density (PPFD) of 62-68 μmol/ m2/s and eight hours of darkness. After this period, groups of shoots were obtained from each meriste-matic apex, which were separated for their subculture four times with 21-day intervals in the P5-0.2 establishment culture medium proposed by López (2006), and in similar temperature and light conditions to have the amount of plant material necessary to establish the embryogenic cell suspensions (ECS).
Establishment of Embryogenic Cell Suspensions from Apexes of Axillary Shoots
To establish the ECS, the apexes of axillary shoots were placed in five 100 ml Erlenmeyer flasks (each with eight explants) that contained 20 ml of the liquid ZZ culture medium proposed by Dhed'a et al. (1991) and modified by (Schoofs, 1997) (Table 1). Each Erlenmeyer flask used constituted a replica, hereinafter Cell Suspension 1, 2 and 3.
Table 1 Culture media used according to the stage of development of somatic embryogenesis in milligrams per liter.
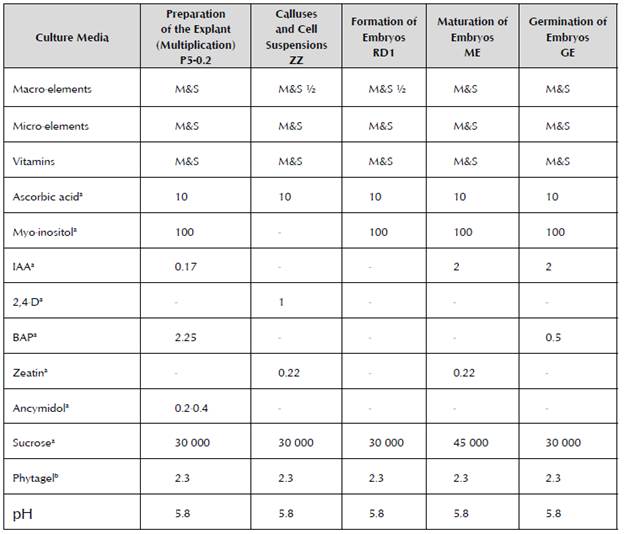
a = Concentration in mg/l; b= Concentration in g/l
M&S: Murashige and Skoog (1962)
The Erlenmeyer flasks were placed in an orbital shaker at 90 rpm and under the system of 16 hours of light at a photosynthetic photon flux density of 62-68 μmol/ m2/s and eight hours of darkness.
During the first months, the changes of culture media were carried out every seven days of culture and consisted of renewing the entire ZZ culture medium. In the third month, these changes were made every three days, renewing 50% of the medium. The meristematic globules formed were taken in the fourth month and subcultured every seven days, renewing 90% of the old culture medium.
After 135 days, the number of embryogenic cell aggregates formed per ml of culture was measured. The counts were made in a 0.1 mm deep Neubauer chamber. To do this, five samples of cell suspensions with a volume of 1.0 ml were taken from each Erlenmeyer flask. The data obtained were analyzed through Dunnett's test. The level of significance used was p ≤ 0.05.
Once established, the cell cultures were screened through metal mesh filters with a hole diameter of 500 Mm. These filtrates constituted the cell suspensions used in a sequence of four experiments conducted: 1) Effect of cell density on multiplication of embryogenic cell suspensions; 2) Formation of somatic embryos; 3) Influence of culture time on the maturation of somatic embryos and their germination; and 4) Conversion of somatic embryos into plants. These experiments are described below.
Effect of Cell Density on Multiplication of Embryogenic Cell Suspensions
Once the cell suspensions were established, the effect of three treatments that corresponded to densities of the initial inoculum (3.0, 6.0 and 9.0%) was evaluated in order to assess the influence of density with respect to the growth process of the cell suspensions and to determine the minimum amount of inoculum that would permit their correct multiplication. The cell multiplication or increase with each treatment was measured every three days according to the evaluation methodology proposed by Schoofs (1997) to measure the settled cell volume (SCV). Five 25 ml Erlenmeyer flasks were used for each evaluated density (3.0, 6.0 and 9.0%). The culture medium and conditions were similar to those used for the establishment of cell suspensions (Table 1), but without removing the culture medium during the 24 days that the experiment lasted. The cell cultures were observed through an optical microscope to determine the cell vitality through fluorescence (Widholm, 1972).
To analyze the growth of the ECS, the nonlinear regression model by Gauss was used through the curve adjustment program CURVE EXPERT 1.3.
Formation of Somatic Embryos
To achieve the formation of somatic embryos, the ECS obtained were adjusted to 12% cell density according to the methodology proposed by López (2006) and they were cultivated in the embryo formation medium RD1 (Dhed'a et al. 1991).
Five Petri dishes were used for each cell suspension analyzed. Four polystyrene meshes with a size of 1.0 cm2 and holes measuring 100 μm were placed over the culture medium in each Petri dish. Then 200 |i at 12% of SCV were added using a micropipette with a cut tip.
The number of formed somatic embryos was measured after 45 days of culture. To take this measurement, 0.1 g of fresh matter (gFM) of the formed somatic embryos was weighed. They were then added to a beaker that contained a mixture of 2.3 g/l Phytagel and water. Then, the mixture was poured onto a Petri dish, left to gel, and divided into quadrants to facilitate counting, which was carried out using a stereoscopic microscope.
To compare the treatments, the analysis of variance was applied, as well as Tukey's range test, with a level of significance of p ≤ 0.05.
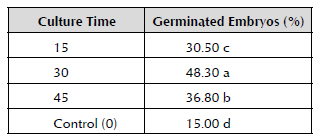
Influence of Culture Time on the Maturation of Somatic Embryos and their Germination
In order to achieve the maturation of the somatic embryos obtained in the previous stage, an experiment was conducted in which the effect of the three culture times (15, 30 and 45 days) on the medium of embryo maturation (EM) proposed by López (2006) was assessed. The embryos were placed at the density of the initial inoculum of 0.5 gFM in five Petri dishes for each studied culture time variant.
To determine the state of maturation of the somatic embryos, their germination was evaluated from a sample of 50 somatic embryos after 15, 30 and 45 days of culture with each one of the studied treatments. To do this, the somatic embryos were placed in different Petri dishes for 30 days, which contained the embryo germination (EG) culture medium proposed by Gómez et al. (2000).
As a control, the experiment required 50 somatic embryos taken from the RD1 medium after 45 days of culture and without passing through the maturation medium. The culture conditions consisted of a temperature of 27 ± 2.0 °C and artificial lighting for a system of 16 hours of light at a photosynthetic photon flux density (PPFD) of 62-68 μmol/m2/s and eight hours of darkness.
To compare the treatment, an analysis of variance was applied, as well as Tukey's range test, with a level of significance of p ≤ 0.01.
Conversion of Somatic Embryos into Plants
In order to achieve the conversion of the somatic embryos into plants, the subsequent survival and growth in environmental ex vitro conditions of the plants originated from somatic embryos were assessed compared to seedlings from propagation by organogenesis (multiplied in the embryos' formation stage), used as a control for in vitro propagation.
This stage was developed according to the methodology proposed by Pérez et al. (1999) under semi-controlled conditions of relative humidity of 85-95% in a lath house covered by a plastic mesh (Saran) with a PPFD of 600 μmol/m2/s.
Polyethylene trays were used with 70 inserts, and a volume of 100 cm3 of sugarcane byproduct. For each origin (somatic embryogenesis and organogenesis), 350 plants with a height of 4-5 cm and two to three leaves were transferred, and planted at a depth of 1.0 cm.
The survival percentage was measured five days after planting, and after 60 days, before transferring them to the field, the phenotypical variables of 60 plants of each origin were evaluated: plant height (cm); length and width of Leaf 2; length of petiole (cm); distance between Leaf 2 and Leaf 3 (cm); as well as the phenotypical variations observed throughout the population according to the methodology proposed by Sandoval et al. (1997).
The statistical analysis of the data was conducted through a hypothesis test for independent samples (student's t-distribution), with a level of significance of p ≤ 0.05.
Results
Establishment of Embryogenic Cell Suspensions from Apexes of Axillary Shoots
Three ECS were established out of the five initiated from the apexes of axillary shoots. No significant statistical differences were found when measuring the number of embryogenic cell aggregates in the ECS (Table 2).
Table 2 Amount of embryogenic cell aggregates present 135 days after establishment of the embryogenic cell suspensions of cv. 'FHIA - 25'.
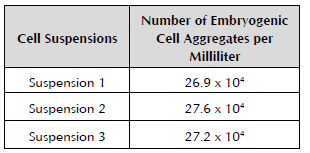
Dunnett's test. NS: Not significant SE ± 0.22.
During the first month, phenolization was observed in the cut area of the explants and later the thickening of them. After two months of culture, yellow globular structures were observed on the surface of the explants, which were separated from these, and started to release embryogenic cell aggregates, as well as the presence of long and vacuolated cells.
Changes were verified in the composition of the ECS, in which it was possible to appreciate small and spherical embryogenic cells with a dense cytoplasmic content, starch granules and a high nucleus tocytoplasm ratio. As the later subcultures were carried out, the amount of isolated and parenchymal cells decreased to almost zero values. These embryogenic cell aggregates came to occupy between 90 and 94% of the cell suspension and their size varied between 80 and 300 pm at 135 days of culture (Figure 1).
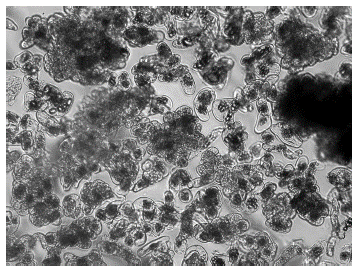
Figure 1 Embryogenic cell aggregates in cell suspensions obtained from the apexes of axillary shoots of cv. 'FHIA - 25' (60x).
The results obtained in this experiment permitted the establishment of ECS from the apexes of axillary shoots placed directly in the liquid culture medium.
Effect of Cell Density on Multiplication of Embryogenic Cell Suspensions
The cell growth curves established from the cell suspensions were initially characterized by a resting phase, because in this phase, the cells adapt to the new culture conditions to later start and increase the speed of cell division during the exponential phase.
The 3.0% cell density facilitated cell increase compared to the growth curves of 6.0 and 9.0% density (Figure 2). In the data obtained using the Gauss model, it was observed that the greatest increase in cell biomass was produced after 23.1 days of culture (0.51 ml) at 3.0% of SCV.
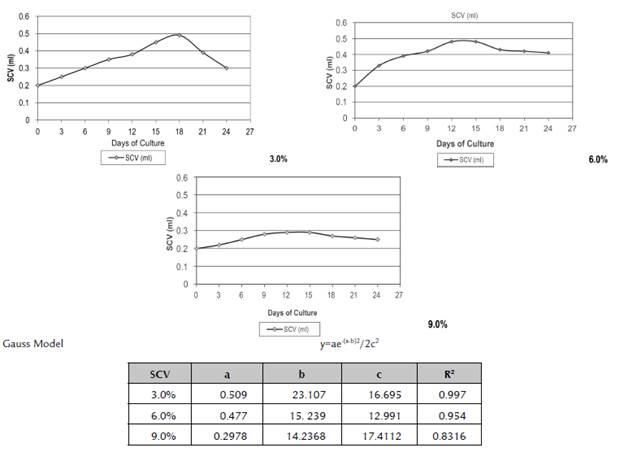
Title: a. Maximum growth of SCV; b. Number of days in which the maximum value of SCV is obtained; c. Variance of the Gauss model.
Figure 2 Influence of cell density on the growth process in cell suspensions established in cv. 'FHIA - 25'.
The same cell density (3%) showed a well-defined and continuous exponential growth phase without impairment of the cell quality (predominance of meristematic cells and cell vitality of 90-95%).
The above demonstrates that during this phase, the cells show intense metabolic activity and achieve their maximum rate of division. This indicates that the subcultures of the cell suspensions should be carried out at a 3.0% cell density of SCV and after 15 days of culture, so that they are maintained in continuous multiplication without passing to the linear phase.
In the 6.0% cell density of SCV, according to the data obtained using the Gauss model, it was observed that the greatest increase in cell biomass was produced after 15.2 days of culture (0.48 ml of SCV). From that date, there was no increase in the cell volume and the cell quality and vitality started to decrease (Figure 2).
The cells established at a density of 9.0% for 21 days of culture did not double their initial volume and the quality of cell suspension, and their vitality sharply decreased. Long cells started to be observed with scarce starch grains.
The right moment to subculture the cell suspensions cannot coincide with the maximum expression of cell density of the culture in suspension, nor with the time in which the main sources of energy run out, but instead with the last exponential growth phase.
Based on the results obtained in this experiment, it could be concluded that the cell suspensions started at a cell density of 3.0% had a better response under the previously explained conditions of management and culture.
Formation of Somatic Embryos
The formed embryos were counted after the embryo-genic cell suspensions had been cultured for 45 days in the RD1 culture medium. ECS 1 and 2 formed a total of 1,580 and 1,610 somatic embryos, respectively, for every 200 μl cultured (Table 3).
Table 3 Number of somatic embryos formed after 45 days of culture in the semisolid RD1 culture medium of cv. 'FHIA -25'.
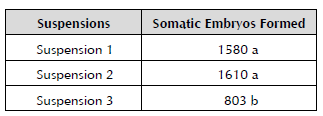
Formed somatic embryos with different letters statistically differ for p ≤ 0.05 according to Tukey's range test.
The results obtained at this stage allowed the initiation of the process of histodifferentiation from embryogen-ic cell suspensions at the cell density of 12.0% to be established as a culture condition for cv. 'FHIA - 25'.
Influence of Culture Time on the Maturation of Somatic Embryos and their Germination
When measuring the incubation time in the EM, it was observed that when the somatic embryos were incubated for 30 days, their maturation was facilitated with the germination of 48.30% of the embryos, showing significant differences to the rest of the studied treatments (Table 4).
Percentages with different letters statistically differ for p ≤ 0.01 according to Tukey's range test.
The mature somatic embryos (Figure 3) showed circular invagination with an opaque center and the presence of a dense meristematic area corresponding to a cordiform state in their development after the embryo evolved asymmetrically to become a cotyledon embryo.

Figure 3 Mature somatic embryos with circular invagination (100x) after 30 days of incubation in the maturity medium.
When the somatic embryos were not previously cultivated in the EM medium, only 15% of them germinated. An initial inoculum density of 0.5 gFM of somatic embryos and 30 days of incubation for the maturation stage were defined from these results.
Conversion of Somatic Embryos into Plants
When assessing the characteristics of the plants originating from the somatic embryos compared to the plants regenerated by organogenesis, a high percentage of survival in the acclimatization stage was observed in both cases. In the case of the plants originating from somatic embryos, it was 98%, and in the organogenesis control, it was 97.4%.
After 60 days in this stage and prior to their transplantation to field conditions, the plants obtained in both regeneration systems did not show phenotypical abnormalities. Regarding the measured variables, except for the plant height, there were significant statistical differences in the other assessed variables in favor of the plants regenerated by somatic embryogenesis (Table 5).
Table 5 Comparison between the plants obtained by somatic embryogenesis and organogenesis after 60 days in the acclimatization stage of cv. 'FHIA - 25'.
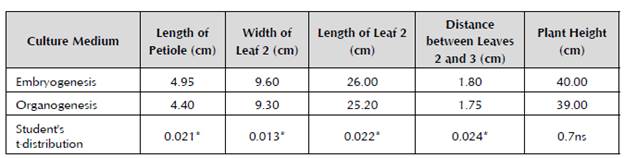
Level of significance for p ≤ 0.05.
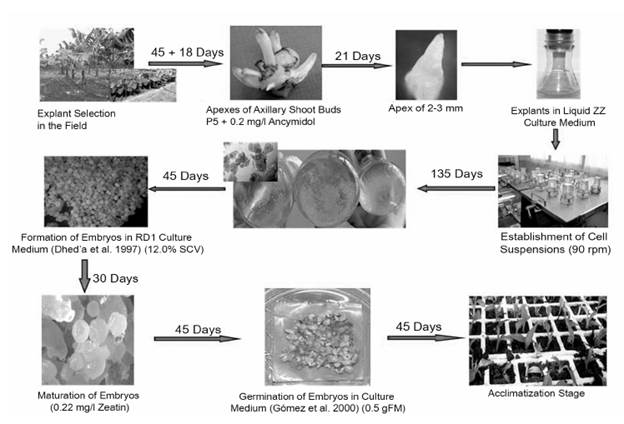
The results obtained from the research allowed a methodology to be prepared for the development of somatic embryogenesis from apexes of axillary shoots in the plantain cultivar 'FHIA - 25' (Figure 4).
Discussion
Establishment of Embryogenic Cell Suspensions from Shoot Apexes of Axillary Buds
The establishment of three ECS of the five initiated from the expiant of apexes of axillary shoots is consistent with the publication by Schoofs et al. (1999), who indicated that not the whole embryogenic complex will provide a good cell suspension. These authors used the multiple buds as a source of explant for the establishment of ECS and obtained a successful rate of one out of two or one out of five. This means that the research conducted corresponds is consistent with the results obtained by other authors.
The formation of yellow globular structures and their later development had also been previously indicated by other authors, such as Dhed'a et al. (1991), who established ECS from "scalps" derived from adventitious shoots (multiple buds) in the Bluggoe cultivar (ABB).
The established ECS are characterized by a high proportion of proliferating embryogenic cell aggregates, the change of color from bright to pale yellow, and the fast settling of cells when the suspension is removed from the orbital shaker, which indicates a high density of cell content, as indicative parameters to identify a good quality ECS (Strosse et al. 2003).
Chong et al. (2005) established cell suspensions directly from explants of immature masculine inflorescences, which permitted homogeneous ECS to be obtained within 20 weeks with a response of more than 70% of the inoculated explants.
Effect of Cell Density on Multiplication of Embryogenic Cell Suspensions
Schoofs et al. (1999) established logarithmic growth curves in ECS in the cell multiplication stage that derived from the multiple bud explant in cv. 'Williams' (AAA). These authors found out that cell densities at 3.0 and 5.0% were higher than at 10%, and in turn, these cell suspensions revealed an almost absent logarithmic phase under these conditions.
Additionally, from the established suspensions of somatic embryos obtained from the cultivation of masculine flowers in cv. 'FHIA - 18' (AAAB), Barranco (2001) achieved the best multiplication at 3.0% cell density of SCV with an exponential growth curve.
Similar results were obtained by López (2006) related to the best response of the 3% cell density of SCV when studying the growth curve in cell suspensions of cv. 'Navolean' (group AAB), with an increase in cell biomass (0.474 ml) after 23 days of culture.
When the growth curves at 6% and 9% of SCV were analyzed, it was observed that regardless of the fact that the cell biomass decreases over time, the quality of the cell suspension decreases as well, making it necessary to increase the number of subcultures. This is corroborated by Georget et al. (2000), alluding to the fact that the ECS loses quality with the number of subcultures and increases the possibility of contamination, decreasing the growth rate and regeneration capacity. This is due to factors including the decrease in nutrients and growth regulators in the culture medium, essentially 2,4-D, as well as the proliferation of thick rapid growth cells rich in starch.
Formation of Somatic Embryos
In cv. 'Bluggoe' (ABB), Dhed'a et al. (1991) obtained the formation of somatic embryos in the liquid culture medium from cell suspensions. Schoofs (1997) obtained 102 to 104 somatic embryos per ml of SCV in semisolid RD1 culture medium from plantain cell suspensions.
In this respect, Arnold et al. (2002) also indicated that it is important to consider that to facilitate the formation and synchronization of somatic embryos, it is necessary to screen the cell aggregates and wash them before their inoculation to eliminate residue from the previous culture medium.
Similar results to the ones obtained in the present research were reported by Barranco (2000) in the Grand Nain (AAA) cultivar with a 15% adjustment in the cell density of the ECS, achieving the process of histodif-ferentiation in the liquid culture medium and forming 2,561.5 ± 95.3 somatic embryos.
When studying several cell densities, Chong et al. (2005) obtained different amounts of somatic embryos, which could have been influenced by the different embryogenic potentials of the cell lines of the Grand Nain cultivar (AAA), all of which corroborates the results obtained in the research conducted.
Influence of Culture Time on the Maturation of Somatic Embryos and their Germination
The cotyledon embryo is characteristic of monocoty-ledonous species as indicated by Natesh and Rau (1984).
Somatic embryos in the globular stage are not ready to germinate and therefore, they need to be placed in a specific medium to facilitate their maturation (Suárez-Castellá et al. 2012), the embryo maturation stage being necessary when cell expansion and the accumulation of reserve substances occurs (Corredoira et al. 2003).
In cv. 'FHIA - 21' (Musa AAAB), Daniels et al. observed that the cell density and time of permanence in the maturation culture medium influence the number of germinated somatic embryos, which was possible to corroborate in the present research.
In cv. 'FHIA - 18' (AAAB), Barranco (2001) established somatic embryos at densities between 0.4 and 0.8 gFM in 250-ml Erlenmeyer flasks, which contained 25 ml of culture medium incubated in darkness and temperature conditions of 27 ± 0.2 °C in an orbital shaker. These presented rapid maturation, which occurred between 15 and 22 days of culture. After analyzing the interaction between the density of the initial inoculum of somatic embryos and the incubation time in the maturation medium in cv. 'Navolean', López (2006) observed that the best combination was the density of 0.5 gFM of embryos during 30 days of culture, achieving a germination percentage of 46.8%. Furthermore, this same author demonstrated that when the somatic embryos were not previously cultivated in the maturation culture medium, only 18.6% of them germinated.
Barranco (2001) achieved 40.6% germination of the hybrid cultivar 'FHIA - 18' in a semisolid culture medium; and Cabrera et al. (2002) achieved 49.3% germination of 'Navolean' (ABB) in the medium proposed by Gómez et al. (2000).
The results obtained by said authors corroborate those obtained in the present research, showing the importance of the maturation stage of somatic embryos to increase their germination percentage.
Conversion of Somatic Embryos into Plants
The efficiency of the embryogenic process of any plant species is a result of the germination and conversion of the somatic embryos into plants, which are essential requirements (López, 2006).
Out of a total of 350 evaluated plants from somatic embryos, a 98% survival rate was achieved compared to the plants regenerated by organogenesis, which obtained 97.4%. Similar results were obtained by López (2006) when studying the conversion of somatic embryos of the Navolean cultivar into plants without the presence of the somaclonal variation in the acclimatization stage. This corroborated the proposal by Sandoval et al. (1997), stating that only around 60% of somaclonal variants can be detected in this stage, which does not implicate that they have not been produced, but that they cannot be visually observed. Therefore, it is necessary to continue the evaluations of these plants in the field until their cycle of development is completed. However, when evaluating the conversion of somatic embryos of the Navolen cultivar to plants, López (2006) observed that 0.5% showed the somaclonal variation characteristics of variegated leaves described by Reuveni and Israeli (1990) as "mosaic-like" axillary shoots.
During the acclimatization stage of regenerated plants of somatic embryos in cv. 'Grand Nain' (AAA), Côte et al. (2000) observed that 0.5 - 1.3% of the population had variegated leaves. They also indicated that when these plants were taken to the field, this morphological abnormality disappeared, perhaps due to the epigenetic changes that had occurred.
Conclusions
The regeneration of plants of cv. 'FHIA - 25' via somatic embryogenesis was achieved from somatic embryos formed at 12% cell density and later matured for 30 days.
The methodology developed fostered the conversion of somatic embryos into plants with 98% survival of them in the acclimatization stage.











 text in
text in