Introduction
Sugarcane (Saccharum spp.) is a nutritional source of carbohydrates (Cova et al. 2006) and one of the main agricultural products in Venezuela in the states of Aragua, Barinas, Carabobo, Lara, Monagas, Portuguesa, Sucre, Tachira, Trujillo, Yaracuy and Zulia. This crop is mainly used as a raw material for the production of sugar and alcohol (Silva et al. 2005). Sugarcane is the crop with the highest global production volume compared to other products of high economic importance according to the data reported as at 2012 by the Food and Agriculture Organization of the United Nations (FAO).
The most cultivated varieties in the country are V7139, V7151, V6410, CC8325, B64129, B8084, PR692176, PR1013, CP721210, CP722086, CP742005, SP711408, SP701284, CL73239, MZC74275, MZC8-25, C32368, C37167, RAGNAR and MY5514, according to the El Palmar Sugar Plant located in the state of Aragua, as well as the varieties C26670, RB855546, V99245, V756, V781, V0050, CC8592 and CC8475 used in this research.
The improvement of this crop is limited by the length of time required to release a new variety for sale (10 to 14 years); and the focus has been on achieving resistance to pests and diseases and on increasing the yields of sucrose and biomass. In order to accelerate the improvement of this important crop, research on the in vitro culture of sugarcane started in the seventies, and since then, it has established different in vitro regeneration systems for many commercial varieties (Gnanapragasam and Vasil, 1990; Suprasanna et al. 2011).
With forty years of research on the in vitro culture of sugarcane, many well-developed systems have been generated, which are routinely applied to research and commercial activities, as follows: (a) micropropagation of genotypes; (b) production of disease-free material from apical meristems; (c) international exchange of germplasm; (d) generation of somaclones; (e) rapid detection of resistance to diseases and pests; and (f) conservation of germplasm. There is a wide range of used and established protocols for the in vitro manipulation of sugarcane morphogenesis and the techniques for mass production of material free from pathogens, which have been integrated into many sugarcane propagation programs (Snyman et al. 2011; Raza et al. 2012).
In somatic embryogenesis, a single cell or a small number of somatic cells are precursors to the formation of a somatic embryo (Silveira et al. 2013). Plant hormones play a critical role in the establishment of somatic embryogenesis. During this process, the plant cells reverse their state of differentiation, acquiring pluripotentiality and determining a new program of development. A critical aspect is the plant hormones' mode of action in the process. Studies indicate that plant hormones participate in the reprogramming of somatic cells to the embryogenic destination and demonstrate a relationship between plant hormones and the molecular pathways that control the process: remodeling of the chromatin, changes in the gene expression pattern, reactivation of the cell division cycle, and regulation and modification of protein synthesis (Thomas and Jiménez, 2005).
To induce somatic embryogenesis it is necessary for there to be a certain concentration of auxins and cytokinins in the medium. Auxins may induce the formation of embryogenic cells and promote repetitive cell division, while cytokinins are necessary for inducing embryogenesis in many species of dicotyledoneae (Abdi and Hedayat, 2011). In general, auxins are defined as compounds with similar biological activity to indole-3-acetic acid, including the ability to promote cell elongation in sections of coleoptiles and stems, cell divisions in cultures of calli in the presence of cy-tokinins, formation of adventitious roots in leaves and separated stems. Although they are chemically diverse, a common characteristic of the active auxins is a molecular distance of 0.5 nm between a fractional positive charge in the aromatic hydrocarbon and a negatively charged carboxyl group (Taiz and Zeiger, 2010). The explant's response to the exogenous addition of auxins and cytokinins is variable and depends on the plant species, state of development of the explant (endogenous level of these hormones) and type and amount of auxins and/or cytokinins.
The process of somatic embryogenesis in different varieties of sugarcane has been studied in order to improve the direct formation of somatic embryos or through a callus phase (Gallo et al. 2000). Oropeza et al. (2001) assessed the potential of the synthetic auxin 2,4-dichlorophenoxyacetic acid (2,4-D) on the embryogenic and non-embryogenic callus production of the PR62258 variety of sugarcane, and they analyzed the related proteins with embryogenic potential in calli and cell suspensions of this culture. Subsequently, Marcano et al. (2002) demonstrated that two Venezuelan varieties of sugarcane responded better to somatic embryogenesis when the calli were induced in media supplemented with 3,6 dichloro-2-methoxyben-zoic acid (dicamba). However, the majority of authors reports that the gramineae respond better to the formation of embryogenic calli with 2,4-D, because it is the most used synthetic auxin, as it affects the growth of plants in a similar way and in the same organs of the auxinic compounds of natural origin.
Therefore, the aim of this research was to analyze the effectiveness of 2,4-D and of dicamba on inducing the process of somatic embryogenesis and the regeneration of vitro seedlings of different varieties of sugarcane, Venezuelan as well as foreign varieties, in order to assess whether the response is due to the genotype of the plant or the action of the auxins considered in the research.
Materials and Methods
Plant Material
Sugarcane plant varieties C26670 (Cuba), RB855546 (Brazil) and V99245 (Venezuela) were used, which were donated by the experimental station INIA (Instituto Nacional de Investigaciones Agropecuarias) located in Yaritagua, Yaracuy State, Venezuela; as well as the varieties V756, V781 and V0050 (Venezuela), CC8592 and CC8475 (Colombia), donated by FUN-DACAÑA (Fundación Azucarera para el Desarrollo, la Producción y la Investigación) located in Chivacoa, Yaracuy State, Venezuela. The most notable agronomic characteristics that permitted the selection of these varieties for our research were: adequate phenological development, germination, lineage and tolerance to diseases.
In Vivo Culture of Sugarcane
The stems of each variety were divided into cuttings with one or two buds, which were incubated in a humid chamber for 24 hours, and then they were individually planted in plastic bags with fertilized soil in nursery conditions with a relative humidity of 70% and an average temperature of 25 °C. When the seedlings reached an appropriate size, from 25 to 30 days after being planted, the explants were removed for establishment of the in vitro culture of all the varieties.
In Vitro Culture of Sugarcane
a) Obtaining Embryogenic Calli
Young stems of plants from the nursery aged from 25 to 30 days were used. From these stems, the external and mature leaves had been removed to leave a section approximately 5 cm long from the last node, wrapped in the youngest leaves. This material was washed with 20% soapy solution, and disinfected with 1% sodium hypochlorite for 20 minutes (Marcano et al. 2002). Two washes with distilled water were carried out in a laminar flow cabinet, each one lasting ten minutes. Then, a last layer of leaves was removed to obtain a stem with a diameter of approximately 1 cm. The explants were divided at a distance of 2 cm to 5 cm from the shoot apex in the form of 2 mm-thick discs for a total of 30 discs per variety. These discs were planted at the ratio of six explants per flask with induction medium, comprised of: complete Murashige and Skoog (1962) salts, 100 mg/l inositol, 1 mg/l thiamine, 0.5 mg/l pyridoxine, 0.5 mg/l nicotinic acid, 2 mg/l glycine, 50 mg/l arginine, 50 mg/l cysteine, 0.15 mg/l citric acid, 30 g/l sucrose, 50 ml/l coconut water, and 8 g/l agar, supplemented with 6.63 mg/l 3,6 dichloro2-methoxybenzoic acid (dicamba) or 3 mg/l 2,4-dichlo-rophenoxyacetic acid (2,4-D), adjusted to pH 5.7 with solutions of 0.1 N NaOH and 1 N HCl. They were cultivated in a room in complete darkness at 25 "C for one month (Marcano et al 2002).
b) Regeneration of Vitro Seedlings
For the development of seedlings, the embryogenic calli were subcultured in a regeneration medium, which is comprised as follows: half Murashige and Skoog (1962) salts, 100 mg/l inositol, 1 mg/l thiamine, 60 g/l sucrose, 200 ml/l coconut water, and 8 g/l agar, adjusted to pH 5.7 with solutions of 0.1 N NaOH and 1 N HCl, and they were incubated in conditions of continuous light in a room at 25 "C for two months (Marcano et al. 2002). Five flasks were planted each with six calli for a total of 30 calli for each variety.
c) Statistical Analysis
The multifactor analysis of variance was used on the interaction between the varieties of sugarcane and the hormones applied with the statistical program Stat-graphics Centurion 12®, with dependent variables (percentage of callus formation and percentage of vitro seedling formation) and independent variables (hormones, varieties and replicas). The aim was to evaluate whether there are significant differences between the percentages of embryogenic callus formation and between the percentages of development of vitro seedlings from the somatic embryos obtained. To identify which of the sugarcane varieties and which of the hormone treatments show significant differences, the comparison of means was applied using Tukey's range test with a confidence level of 95%.
Results and Discussion
In Vitro Culture of Sugarcane
The disinfection protocol was 100% efficient, because no contamination was observed in any of the culture phases. The procedures of in vitro culture and propagation were carried out in a room with adequate aseptic conditions equipped with a laminar flow cabinet, incinerator, sterile instruments and duly sterilized culture media. This achievement is extremely important if for example, this result is compared with the results obtained by Mittal et al. (2009), who reported the need to use 500 mg/l cefotaxime, an antibiotic belonging to the family of third-generation cephalosporins, as a promoter of somatic embryogenesis and the subsequent regeneration of shoots of different varieties of Indian sugarcane, and to maintain asepsis during the morpho-genetic process. Kour and Kapoor (2015) found that this antibiotic did not promote somatic embryogenesis in the sugarcane varieties CoJg4, CoJ83 and CoJ86. There was no need to use any kind of antibiotic in this research, which could interfere with the morphogenesis of the somatic embryos, as well as the effect on the costs of establishment of the culture.
a) Obtaining Embryogenic Calli
Different genetic and physiological factors influence the induction of somatic embryogenesis, leading to the replacement of the gene expression pattern existing in the explant's tissue with a new expression of embryogenic genes. This process is only possible if the cells are competent and receive the appropriate inducing stimuli. Among the influencing factors, plant hormones, especially auxins, are key components, because their exogenous application recapitulates the embryogenic potential of the mitotically inactive somatic cells. Several studies have indicated that signposting by auxins, as well as stress, can be key events in genetic reprogramming of somatic cells during early embryogenesis, which is necessary for de-differentiation and to acquire the embryogenic condition (Silveira et al. 2013).
The different stages of the process of somatic embryo-genesis in the V99245 sugarcane variety can be observed in Figure 1, representing all the varieties studied in this work. In Part A of the figure, the inoculated leaf discs can be observed in the callus induction medium. After 25 days, in Part B of the figure, the formation of a compact callus can be observed with a whitish color; typical characteristics of the embryogenic callus of sugarcane (Oropeza et al. 2001). Taylor et al. (1992) provide a precise description of the different types of callus that originate from segments of young sugarcane leaves: Type I appears first, after five days of culture at the ends of the cutting and it is characterized by being semi-translucent and consisting of long, large cells. Type II is developed in the tissue of Type I after 13 days of culture, and it is smooth, slimy, yellow and contains highly disassociated round, vacuolated cells. Type III appears after 13 to 21 days at the ends of the explants around the vascular tissue, consisting of a compact, hard callus with globular surface nodules, which is yellowish at the start but then turns a whitish color and is comprised of small, round cells with thick cytoplasm. Type IV is yellow, friable, presents agglomerates of semi-organized structures, and consists of small, round cells with thick cytoplasm. Type I and Type II calli are non-morphogenic, while Type III and IV calli can regenerate plants at a high frequency through somatic embryogenesis, and at a low frequency through organogenesis, respectively. The Type III callus is referred to as an embryogenic callus and the heterogeneity is due to the explant coming from the transversal section of different leaves with different degrees of maturity. Guiderdoni and Demarly (1988) described only two types of callus: the friable callus and the compact callus, which apparently coincide with Type I and Type III of Taylor et al. (1992). Similar results were obtained by Oropeza et al. (2001) and by Marcano et al. (2002), only observing the compact and friable calli in accordance with the description by Guiderdoni and Demarly (1988). Silveira et al. (2013) studied the differences in the acquisition of competencies and the later maturation of the somatic embryo in embryogenic calli and non-embryogenic calli of the SP791011 variety of sugarcane through histological analysis, growth curves, number of somatic embryos, and polyamide content. The embryogenic calli could be distinguished from the non-embryogenic calli because they presented round tissue with a dry and compact aspect, and an opaque, whitish color with less oxidation, as well as the appearance of green spots after several days; while the non-embryogenic calli presented long, smooth and friable cells, were mucilaginous, and had a translucent brown color due to oxidation in the presence of light.
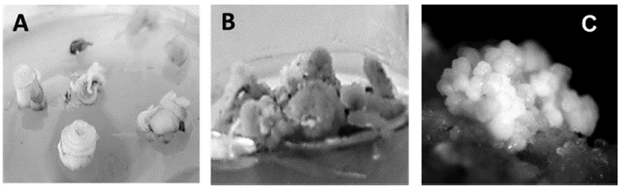
Figure 1 Induction of somatic embryogenesis with dicamba in the V99205 variety of sugarcane. A) Discs of surrounding leaves at the start of induction. B) Callus generated after 25 days of culture in induction medium. C) Calli showing embryogenic cell aggregates after 32 days of incubation in induction medium (110X).
In Figure 1C, the aggregates of embryos formed from the embryogenic calli are observed 32 days after planting, showing the characteristic whitish color. The small amount of non-embryogenic callus obtained presented a smooth and friable aspect, showing cell oxidation with translucent brown coloring. The embryogenic calli showed a more compact aspect, and an opaque and whitish coloring with numerous somatic embryos in their periphery, which after five days started to germinate, observing a large amount of stems and roots. The rest of the varieties showed the same type of growth. Although no histological study was conducted to determine the embryogenic origin of the vitro seedlings, the culture conditions and the morphology of the callus obtained were similar to those reported by Oropeza et al. (2001) and by Marcano et al. (2002), who through an anatomical study, corroborated the observations at a morphological level in the embryogenic calli. Therefore, given the morphological characteristics of the calli obtained in this research and the high frequency of regeneration of seedlings from these calli, it is very probable that the main process of in vitro regeneration that is occurring is embryogenic.
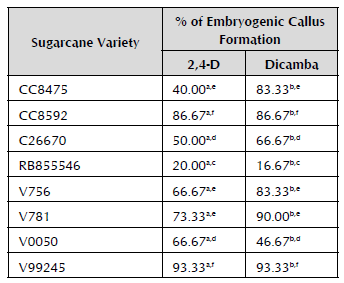
In Table 1, the percentages of formation of embryogenic calli for each variety are observed with both hormone treatments, which show significant differences between 2,4-D and dicamba. In terms of the response by variety, a particularly low response is observed for the RB855546 variety with both treatments, which corroborates the fact that every embryogenic response depends on the genotype. In general, for the V0050 variety, the formation of embryogenic calli is greater with 2,4-D. A very similar response was observed between the varieties CC8475, V756 and V781, but the response oscillates between 40 and 90%, all with a greater response to dicamba; just like between the varieties CC8592 and V99245. These last two varieties have the highest response for the formation of embryogenic calli with both treatments.
Comparison of means with Tukey's range test with a confidence level of 95%. Letters a and b indicate highly significant differences between the hormone treatments. Letters c, d, e and f represent highly significant differences between the varieties' responses in callus formation. Varieties with the same letter do not present significant differences between them.
The start and maintenance of embryogenic callus is an important step for the development of somatic embryos and regeneration of seedlings (Silveira et al. 2013). Traditionally as well as currently, somatic embryogenesis is induced with 2,4-D in many species of gramineae. For the first time, Ho and Vasil (1983) reported that calli were formed in the cut ends of monocotyledon explants cultivated in Murashige and Skoog (1962) medium supplemented with 2,4-D (Guiderdoni and Demarli, 1988; Guiderdoni et al. 1995; Suprasanna et al. 2005; Lakshmanan et al. 2005; Raza et al. 2012;
Taparia et al. 2012; Kaur and Kapoor, 2015). In our research, the induction of embryogenic calli and somatic embryos was slightly greater in the explants cultivated in media supplemented with dicamba (70.83% of embryogenic callus per variety), although the majority of the varieties responded to 2,4-D (62.08% of embryogenic callus per variety). The percentage of embryogenic callus formation of each variety for each treatment is observed in detail in Table 1. Rapid development of embryogenic callus was observed during the induction phase under the test conditions in culture media supplemented with dicamba, a compound that is used in field conditions as a selective herbicide. Its mode of action is to modify the transport of indole-3-acetic acid, destroying the cambium and the parenchyma at the height of the nodes or above them. However, its chemical structure is that of an artificial auxin, which when used for in vitro cultures of sugarcane, permits the anaplasia of the tissue in the discs of surrounding leaves.
The use of explants of young leaves in our research has been validated in different protocols established for the in vitro morphogenesis of sugarcane using explants of different parts of the plant, including for example, meristems, whorls of internal leaves, segments of inflorescences, immature inflorescences, segments of young leaves, and discs of immature leaves. However, the most used one is the disc of immature, surrounding leaves close to the apical meristem inoculated horizontally, with its distal ends in contact with the medium, because the best results in the production of somatic embryos have been achieved with this kind of explant (Guiderdoni et al. 1995; Oropeza et al. 2001; Marcano et al. 2002; Suprasanna et al. 2011; Joshi et al. 2013). Kaur and Sandhu (2015) established an efficient system for the direct production of adventitious buds, also from the discs of young leaves, demonstrating the potential of this explant for mass industrial production of sugarcane plants.
Manchanda and Gosal (2012) studied the effect of activated carbon, the sources of carbon and gelling agents on the somatic embryogenesis of sugarcane, and largely recommend the addition of activated carbon to the culture medium to reduce the blackening of the tissue by the exudation of phenolic compounds, and therefore, increase the embryogenic potential of the tissue. Additionally, they recommend supplementing the medium with maltose, although the use of sucrose continues to provide good results. Finally, they recommend gelling the culture media with agar, because they observed that with its use, the formation of somatic embryos increased, as well as the regeneration frequency of sugarcane compared to Gelrite. Blackening was not observed, so it was not necessary to use activated carbon. The small amount of oxidation observed was controlled with the addition of cysteine to the culture medium.
Suprasanna et al. (2005) found that embryogenic callus formation was higher with the combination of 2,4-D and zeatin in the Co91010 variety, while for the CoC671 variety, this combination was made efficient by adding proline to the culture medium. Upon analyzing the response obtained in our research, particularly with the RB855546 variety, it could be recommended to supplement the M&S (1962) salts used for inducing embryogenic calli (which contain the amino acids arginine and cysteine) with proline in order to improve the response of this variety. Furthermore, no synthetic cy-tokinins were used in our research in combination with the auxins tested for inducing the process of somatic embryogenesis. Apparently, the amount of zeatin that the coconut water contains is sufficient to induce the processes of cell division and elongation, which in combination with the auxins, provide the cytokinins during this process.
Marcano et al. (2002) optimized the process of somatic embryogenesis in Venezuelan varieties of sugarcane, using the synthetic auxins 2,4-D and dicamba, both of which are substances used in the field as herbicides, because they affect the transport of natural auxins and cell elongation. They demonstrated that after 45 days in the medium supplemented with dicamba, the highest percentage of embryogenic callus formation was observed for the tested Venezuelan varieties V756 and V781.
As previously mentioned, numerous authors have reported that somatic embryogenesis in sugarcane is induced by the auxin 2,4-D (Ho and Vasil, 1983; Brisibe et al. 1994; Falco et al. 1996; Oropeza and García, 1996; Oropeza et al. 2001; Watt et al. 2009; Snyman et al. 2011). However, in the majority of the varieties analyzed in this research, the embryogenic callus percentage obtained is greater when dicamba is used. This may be due to the structural difference of the molecules of 2,4-D and dicamba, as the latter can be absorbed and degraded by the plant tissue more quickly.
b) Regeneration of Vitro Seedlings
The efficient regeneration of plants through an in vitro culture system is one of the most important stages in the process, because it determines the efficiency of the process. Furthermore, it is an essential prerequisite for a complete protocol of genetic transformation (Suprasanna et al. 2011; Manchanda and Gosal, 2012).
Figure 3 shows the development of the sugarcane seedlings of the V99245 variety once the calli were transferred to the regeneration medium, which does not contain plant hormones. Figure A shows the formation of stems five days after having been transferred to the regeneration medium, where an average of approximately 80% regeneration of plants from the calli obtained could be quantified (Table 2). The progress of the culture can be seen in Figure B3 through observation of the elongation of the stems and the emergence of the first roots after 15 days of cultivation in the regeneration medium. This is followed by the increase in the amount of stems and roots formed after 20 days, which can be appreciated in Figure C.
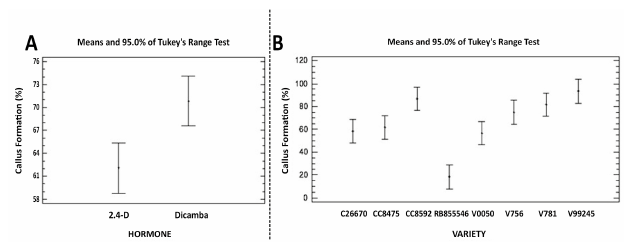
Figure 2 Graphs showing analysis of homogeneity of variance with a 95% confidence level through comparison of means by Tukey's range test for embryogenic callus formation in sugarcane. A. Hormone treatments. B. Varieties of sugarcane.
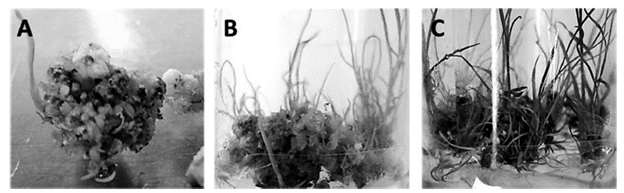
Figure 3 Formation of sugarcane plants, V99245 variety, in regeneration medium. A) Formation of stems after five days of culture. B) Elongation of stems and formation of roots after 15 days of culture. C) Elongation of stems and roots after 20 days of culture in regeneration medium.
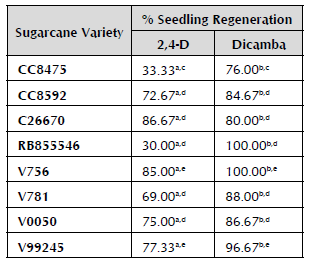
Table 2 shows the values obtained from the regeneration of seedlings with embryogenic calli from the hormone treatments with 2,4-D and dicamba, where dicamba is the most efficient hormone, because the calli generated in this treatment present a high percentage of regeneration of complete seedlings. In terms of the differences between varieties, three groups with highly significant differences can be distinguished. The first group is only comprised of the CC8475 variety, which presents a very high response to the treatment with dicamba and a very low response to the treatment with 2,4-D. The second group, comprised of the CC8592, C26670, RB855546, V781 and V0050 varieties, does not present significant differences between the varieties, highlighting that they all respond better to dicamba. The last group, comprised of the V756 and V99245 varieties, also presents a better response to dicamba. However, its response to 2,4-D is not low.
Comparison of means with Tukey's range test with a confidence level of 95%. Letters a and b indicate highly significant differences between the hormone treatments. Letters c, d, e, and f represent highly significant differences between the varieties' response in the regeneration of complete seedlings. There are no significant differences between the varieties that have the same letter.
The RB855546 variety did not present significant differences in terms of callus formation from the hormone treatments with 2,4-D and dicamba, and the response values for these treatments were lower than in the other varieties. However, when transferring the embryogenic calli of this variety to the plant regeneration medium, the response in terms of formation of complete seedlings was significantly higher with dicamba. This variety of sugarcane originating from Brazil needs to be studied in more detail to be able to establish an efficient system of somatic embryogenesis.
The differential response of each variety to each hormone treatment in the regeneration of complete sugarcane seedlings can be seen in Figure 4, where the greatest percentage of seedling regeneration from the calli induced with dicamba and the grouping of the aforementioned varieties can be clearly observed.
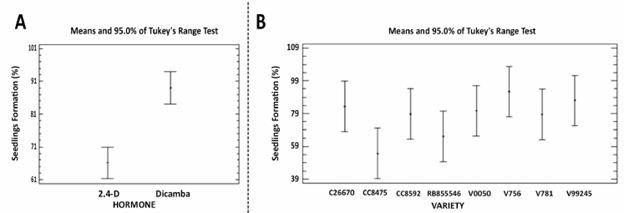
Figure 4 Graph of analysis of homogeneity of variance with a 95% confidence level through comparison of means by Tukey's range test for the regeneration of complete sugarcane seedlings. A. Hormone treatments. B. Varieties of sugarcane.
Through a collective analysis of the induction phase of the callus formation process and the development phase of the somatic embryos and regeneration of vitro seedlings, we can conclude that the treatment with dicamba is 90% more efficient. Highly significant differences can be observed between the hormone treatments. However, it can also be observed that according to its genotype, each variety presents an individual response, despite the fact that the best results are generally obtained with dicamba. Marcano et al. (2002) demonstrated the high capacity of regeneration of the calli obtained in the induction medium with dicamba when transferring them to the regeneration medium (without hormones), where after four days, the first stems could already be observed, and after 45 days, complete sugarcane plants of the V756 and V781 varieties had been regenerated. We obtained similar results in our research, observing the first stems after five days and the regeneration of complete plants after 45 days.
According to our results, the "dicamba" treatment was more effective than the 2,4-D treatment to induce embryogenic calli and the regeneration of calli in six out of the eight varieties studied. Similar results were obtained by Filippov et al. (2006) with different wheat cultivars, where exposure to dicamba resulted in the quicker development of somatic embryos from callus compared to 2,4-D or picloram. Some authors have reported this effect of dicamba on gramineae, specifically in cereals, and explain that this synthetic growth regulator has a fast metabolism in the tissues, while 2,4-D is a very stable auxin that shows strong resistance to degradation and enzyme conjugation in plant cells. Exogenous auxins are necessary to induce mitotic cell division and elongation during the callus induction phase. However, they must be eliminated from the culture medium once said cell divisions have occurred so that embryogenic morphogenesis can proceed. This is when dicamba is presumably degraded more quickly in the callus tissue than 2,4-D, because the germination of the somatic embryos occurs earlier and more efficiently in the medium supplemented with dicamba (Filippov et al. 2006). A strong influence of the genotype has been observed between many kinds of explants and many species. These differences have been associated with the variations in susceptibility between the different genotypes to the genetic programming and reprogramming of the embryogenically competent cells (Filippov et al. 2006). Similarly, Kaur and Kapoor (2015) demonstrated the influence of the genotype on the formation of somatic embryos in the CoJ64, CoJ83 and CoJ86 varieties of sugarcane.
Finally, the results of this research permit the recommendation of the use of dicamba for inducing the somatic embryogenesis process in different sugarcane varieties and the regeneration of vitro seedlings in culture media without growth regulators. The established protocol ensures the availability of sufficient material, and most importantly, material free from pathogens, under completely controlled environmental conditions to conserve the germplasm of sugarcane varieties with different geographical origins and to apply advanced techniques to improve this important crop.
Conclusions
The statistical analysis demonstrated that there are highly significant differences between the hormone treatments, with dicamba being the most efficient growth regulator for the induction of somatic embryo-genesis in sugarcane.
The response of sugarcane varieties to hormone treatments is genotype dependent. The V99245, CC8592, V781 and V756 varieties showed the best response to the process of inducing somatic embryogenesis with dicamba. The V756, C26670, V99245 and V0050 varieties reached the highest percentages of regeneration of vitro seedlings.
This research established an efficient system of somatic embryogenesis in sugarcane varieties from different countries' improvement programs.
Perspectives for Future Research
Confirm the use of dicamba as the most efficient synthetic auxin for establishing somatic embryogenesis in all the Venezuelan varieties of sugarcane.
Include all the Venezuelan sugarcane varieties in a germplasm bank in order to create a national seed certification program, making the most of the in vitro culture system.











 texto em
texto em