Introduction
Pseudomonas fluorescens is a bacterium with high potential for the biological control of different phyto-pathogenic microorganisms due to its multiple mechanisms of action, such as inducing systemic resistance, production of siderophores, competition for space and nutrients, and the production of different metabolites. Out of these metabolites, pyoluteorin, 2,4-diacetylphloroglucinol (DAPG), pyrrolnitrin, hydrogen cyanide and other metalloenzymes are of note, (Loper & Gross, 2007; Anjaiah, 2006; Manikandan et al. 2010; Siddiqui & Shaukat, 2003). Some of the diseases over which P. fluorescens exercises control are sheath blight in rice (Oryza sativa L.) caused by Rhizoctonia solani (Commare et al. 2002; Rabrindran & Vidhyasekaran, 1996); damping-off in tomatoes (Solanum lycopersicum L.) caused by Fusarium oxysporum (Manikandan et al. 2010; Valencia et al. 2005); bacterial spot in tomatoes caused by Xanthomonas campestris pv. Vesicatoria (Kamal et al. 2008); rotting of the fruit and mold in chilies caused by Colletotrichum capsici and Leveillula taurica, respectively (Anand et al. 2010); and wilt caused by the soil fungus Verticillium dahlia, a disease responsible for great economic losses in crops such as cotton (Gos-sypium sp. L) (Erdogan & Benlioglu, 2010).
Like other microorganisms, P. fluorescens is susceptible to different environmental conditions, particularly temperature and pH (O'Callaghan et al. 2006). Therefore, for its efficient implementation as a biological control tool, it is necessary to develop products with a formulation that ensures their effectiveness and stability during storage (Burges, 1998).
There are many products around the world with P. fluorescens as the active ingredient that are formulated for the biological control of different plant pathogens (Hernández-Rodríguez et al. 2008; Hernández-Rodríguez et al. 2014). These products include BioCure-B®, recommended for the control of Mycosphaerella grasicola, Pythium spp., Rhizoctonia solani, Fusarium spp., Botrytis cinerea, Sclerotium rolfsii, and Sclerotinia homoeocarpa (T-Stanes & Company Limited, s.f), and BlightBanA506® formulated as a wettable powder for the control of Erwinia amylovora in potato (Solanum tuberosum L.) and tomato crops (Copping, 2009), among other products.
The autochthonous strain P. fluorescens Ps006 has the capacity of producing biosurfactants, a phosphorus solubilizing activity, and a zoosporicidal activity, as well as the potential for biological control of pathogens by showing a reduction in the severity index (SI) of O. virulentus (SI of 0.6) in lettuce plants (Lactuca sativa L.) compared to the control pathogen (SI of 4.7). Furthermore, this bacterium applied at a concentration of 1 x 107 cells per ml facilitated and significantly increased the length and width of the leaves, the dry biomass of the aerial part, as well as the development in the length and dry biomass of the root of fique plants, demonstrating a promoting effect on plant growth (Smith et al. 2013). Taking said characteristics into account, this research aimed to develop a prototype for the formulation of wettable powder (WP) using P. fluorescens Ps006 as an active ingredient, and to select the most suitable packaging atmosphere to maintain the viability of the microorganism.
Materials and Methods
Microorganism and Conservation
The isolation of P. fluorescens Ps006 was used, obtained from the rhizosphere of fique Furcraea andina (Trelease 1808) in the municipality of Totoró (2°38' N and 2°15' W, at 2,750 m.a.s.l and 14 °C), in the Cauca Department, Colombia (Sastoque, 2010). The microorganism was conserved in saline solution (0.85% NaCl) with 10% glycerol and 0.10% peptone at -70 ± 2 °C.
Production and Characterization of the Active Ingredient of P. fluorescens Ps006
P. fluorescens Ps006 was planted in Luria Bertani (LB) agar medium (Oxoid CM1021) and was incubated for 48 hours at 28 ± 2 °C. From this culture, a cell suspension was prepared in Tween 80 at 0.5%, and it was inoculated in a 250-ml Erlenmeyer flask with 100 ml of LB medium. The concentration of the suspension was estimated by reading the absorbency at a wavelength of 300 nm, and said value was extrapolated in a previously standardized calibration curve. Fermentation was carried out using constant agitation of 175 rpm at 28 ± 2 °C for 48 hours.
Three fermentation batches were developed, which were characterized by determining the cell concentration in colony-forming units per milliliter (CFU/ml), the pH and the in vitro biological activity, three times for each batch.
The bacterial concentration was determined through a count in a Petri dish, planting three decimal dilutions of the fermentation broth three times in Petri dishes with LB medium and incubating them for 24 hours at 28 ± 2 °C, when the CFU/ml count was carried out. Once the normality (Shapiro-Wilk test 95%) and homogeneity of variance (Bartlett's test 95%) of the data was verified, an analysis of variance (ANOVA) and Tukey's test were carried out with a confidence level of 95%. The Statistix 8.0 statistical program was used.
The pH of the three fermentation batches was measured with a previously calibrated Hanna® Instruments brand potentiometer. The standard deviation and the coefficient of variation of the data were determined.
The in vitro biological activity was estimated as the growth inhibition of four recognized plant pathogens (Raut et al. 2012) through the dual culture technique (Shi et al. 2014). To do this, the strains F. oxysporum MAP5 isolated from Physalis (Physalis peruviana L.); R. solani Rh200 isolated from potato (Solanum tuberos-um); B. cinerea Bc008 isolated from blackberry (Rubus sp. L.); and Sclerotinia sclerotiorum Sc021 isolated from potato (Solanum tuberosum) were used. To determine this, they were planted in the surface of PDA agar medium samples of 100 μl of the three fermentation batches and placed in a central point of the medium, inoculating a disc with a 5 mm diameter in the culture medium grown with the plant pathogen fungi aged eight days. Three experimental units were used (Petri dishes) for each treatment. The control consisted of PDA agar medium without a bacterial inoculum, but inoculated with plant pathogen fungi as previously described. The Petri dishes were incubated for eight days at 25 ± 2 °C. After this time, the mean diameter growth of each colony was determined by calculating the average length of four straight lines drawn from one end to another of the colony, passing through its center and dividing it into eighths (Pradeep et al. 2013).
Selection of Compatible Solid Supports
Three solid supports in powder were evaluated, called S1 (clay), S2 (silicate) and S3 (stearate), each one in two different conditions of humidity (10% and 20%), and inoculated with the active ingredient at a concentration of 1 x 109 CFU/ml. The humidity of the supports at 10% was provided by the mixed active ingredient, while to adjust the 20% humidity and maintain the same cell concentration, a pH 7.5 buffer solution was added. Subsequently, 1-g samples of support inoculated with the active ingredient were added to previously sterilized glass vials, which were sealed with rubber stops and metal clips, and were stored at different temperatures (8 ± 2 °C, 18 ± 2 °C and 28 ± 2 °C). Before starting storage, and after one, two and three months, the viability of P. fluorescens Ps006 was evaluated using the previously described method of counting in a Petri dish (results expressed in CFU/g). The sample contained in each vial was mixed with 9 ml of Tween 80 at 0.5%, and shaken to be later planted in the LB culture medium. A completely randomized design was used with measures repeated over time. Two samples of each treatment were evaluated three times in each measurement time. The treatments with which the greatest viability and stability of the active ingredient was obtained in the three months of storage were selected.
Evaluation of Adjuvants and Packaging Atmosphere
Once the supports compatible with the active ingredient were selected, the effect of the presence or absence of air in the packaging and the effect of skimmed milk and glycerol (adjuvants) were evaluated, which are commonly used as stabilizers of the microorganisms' viability during their preservation (Burges, 1998). The selected supports were mixed with the adjuvants at 5% and were inoculated with the bacterial culture, adjusting it to the selected humidity. The inoculated supports without the addition of adjuvants were used as a control. The samples were stored under different atmospheric conditions: vacuum-packed or with the presence of oxygen, and at temperatures of 8 ± 2 °C, 18 ± 2 °C or 28 ± 2 °C. The effect of these conditions was evaluated over six months. For the treatments stored with oxygen, 1-g samples of the support inoculated with the active ingredient were dispensed in previously sterilized glass vials, which were sealed with rubber stops and metal clips. For the vacuum-packed storage, 1-g samples of the support inoculated with the active ingredient were deposited in PET (polyethylene terephthalate) bags, and vacuum sealed with a Van der Stahl Scientific® packaging machine. The viability of the bacteria was determined through a count in the Petri dish, planting three decimal dilutions three times in the surface in Petri dishes with the LB agar medium.
A completely randomized design was followed with measures repeated over time, and the feasibility of two samples of each treatment was determined three times in each evaluation time.
Statistical Analysis
The normality and homogeneity of variance were determined through the Shapiro-Wilk test (95%) and Bartlett's (95%) test, respectively. Once these principles were demonstrated, an analysis of variance (ANOVA) and a Tukey's range test (95%) were applied. The Statistix Version 8.1 program (Analytical software, Florida, USA) was used.
Results and Discussion
Production and Characterization of the Active Ingredient P. fluorescens Ps006
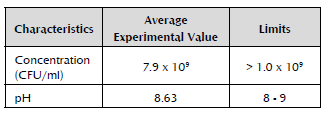
An average pH of 8.63 was obtained for the three active ingredient batches with a 0.2% coefficient of variation. The cell concentrations for each one of the evaluated batches were 6.73 x 109, 8.07 x 109 and 8.93 x 109 CFU/ml. The coefficient of variation of the evaluation results was 0.6%, and there were no significant differences between the results of the batches (F = 2.06; DF = 2; p = 0.2081), which indicates low variability and high repeatability in the production of the active ingredient (Villamizar et al. 2005). The pH oscillated between 8.60 and 8.65, finding no significant differences between the results of the evaluated batches (F = 3.74; DF = 2; p = 0.0882).
Based on the results obtained for each parameter, ranges or limits of acceptance were proposed in this study for the characteristics of the active ingredient based on Ps006, which will be used as a reference for its quality control (Table 1).
In Figure 1, the in vitro effect of the active ingredient on the four evaluated pathogens is observed with inhibitions of the diameter growth of R. solani Rh200, B. cinerea Bc008, S. sclerotiorum Sc021 and F. oxysporum MAP5 between 93.64% and 36.80%. The antagonistic activity of P. fluorescens toward pathogens that affect crops of economic importance has been demonstrated by several authors. For example, for F. oxysporum, a control of 81.21% was achieved in field conditions (Manikandan et al. 2010), and for R. solani, decreases of 42% were observed in the intensity of the disease (Rabrindran & Vidhyasekaran, 1996) and of 47.89% (Commare et al. 2002) in greenhouse conditions. Likewise, a 67.7% decrease in the in vitro growth of Botrytis sp. was achieved (Mikani et al. 2008), as well as a 26.3% reduction in the incidence of the disease for S. sclerotiorum in field conditions (Fernando et al. 2007). These results confirm the potential of P. fluorescens as a biological control agent and demonstrate the potential of its use as an active ingredient in biopesticides for the control of pests that affect crops of economic importance. However, research on the scale of plant pots and fields are necessary to demonstrate this hypothesis.
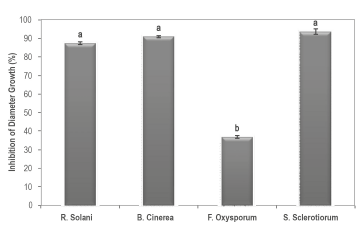
Figure 1 Antagonistic in vitro activity of the active ingredient based on P. fluorescens Ps006, determined as the diameter growth inhibition of four plant pathogenic fungi. Treatments with the same letters do not present significant differences according to Tukey's range test (95%).
The most resistant pathogen to the antagonistic activity of P. fluorescens Ps006 was F. oxysporum MAP5 with an inhibition of the diameter growth of only 36.80%, a significantly lower value (F = 14.9; DF = 3; p = 0.0000) to those obtained with the other evaluated pathogens, which presented inhibition percentages above 87%. This could be due to a greater resistance in the cell wall of the F. oxysporum mycelium; a microorganism that has polymers of heteroglucans that are not found in the other evaluated pathogens, which have been associated with the resistance and maintenance of cell integrity (Nuero, 1995). It is also possible that the isolation of F. oxysporum MAP5 has the capacity to inhibit the biosynthesis of 2,4-diacetylphloroglucinol (2,4-DAPG); an antibiotic with a wide spectrum that is recognized as one of the main mechanisms of action in a wide range of isolations of P. fluorescens (Showkat et al. 2012) . This behavior was demonstrated by Schouten et al. (2004), who evaluated the sensitivity of 41 isolations of F. oxysporum to said antibiotic, and found resistant isolations of 17%, which attributed to the capacity of this pathogen to produce fusaric acid, a powerful inhibitor of the biosynthesis of 2,4-DAPG.
The mode of action of P. fluorescens as an antagonist of plant pathogens has been related to the competition for space and nutrients. This phenomenon was observed by Commare et al. (2002), who determined the antagonistic activity of formulations of P. fluorescens in talc, showing 47.89% control of R. solani in greenhouse conditions, the results being attributed to competition for space. In the study conducted by Valencia et al. (2005), it was concluded that the activity of P. fluorescens ZUM80 on F. oxysporum was due to competition for nutrients, depriving the fungus of the iron available in the medium through the production of siderophores. This antagonistic activity was facilitated when the bacteria had pre-exposure time in the medium (Valencia et al. 2005). Apart from these mechanisms of competition in studies conducted by Khanam, Ueno, Kihara, Honda & Arase (2005), it was found that the salicylic acid produced by P. fluorescens inhibits the formation of infection structures such as the germ tube and appressoria in B. cinerea, which suggests that this bacterium also has the capacity to produce other metabolites with effective antagonistic activity.
Selection of Compatible Solid Supports
The total loss of viability of the active ingredient after three months of storage at the three evaluated temperatures is shown in Figure 2. For the S1 support at 10% humidity (S1-10%), losses between 14% and 31.9% were observed, and for S1 at 20% humidity (S1-20%), losses between 9.6% and 33.2% were observed. For the S2 support at 10% humidity (S2-10%) at the three temperatures, a 67.7% loss of viability was observed, and for S2 at 20% humidity (S2-20%), the reduction in the viability oscillated between 2.3% and 9.4%. Finally, for the S3 support at 10% humidity (S3-10%) at the three temperatures, a 78.1% loss was observed, and for S3 at 20% humidity (S2-20%), the loss of viability oscillated between 25.7% and 69.6%.
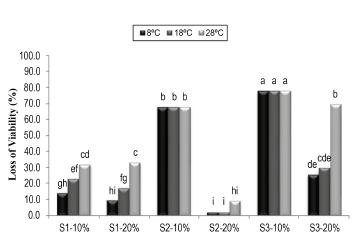
Figure 2 Total loss of viability of the active ingredient of P. fluorescens Ps006 mixed with the solid supports after three months of storage at three temperatures. S1-10%: Support 1 at 10% humidity; S1-20%: Support 1 at 20% humidity; S2-10%: Support 2 at 10% humidity; S2-20%: Support 2 at 20% humidity; S3-10%: Support 3 at 10% humidity; S3-20%: Support 3 at 20% humidity. Treatments with the same letter do not present significant differences according to Tukey's test (95%).
The results showed that the 10% and 20% humidity conditions, to which the systems were adjusted for their storage, had an effect on the stability of the Ps006 bacterium's viability. The loss of viability with S2 and S3 at 10% was significantly higher than for the systems with 20% humidity (F = 402; DF = 17; p = 0.0000) (Figure 2). This may be because the treatments with 20% humidity required the addition of a volume of buffer solution at pH 7.5 to adjust the humidity. This possibly exercised control on the ionic state of the formulation, making it less susceptible to abrupt changes in pH, which may affect the stability and integrity of the cell membrane, cause the denaturation of enzymes, and alter the ionic interactions that allow them to recognize and bind to the substrate (Calvo et al. 2004; Nelson & Cox, 2005).
The greatest losses in viability obtained with S2 and S3 when the humidity was adjusted to 10% could be because at said humidity, the concentration of solutes in the system is greater compared to the treatment at 20% humidity. This hypertonic atmosphere in the medium at 10% humidity could have caused plasmolysis phenomena, forcing the cell to lose water, which increases the viscosity of the intracellular environment, decreasing its volume, causing a retraction of the cell membrane, and subsequently, cell death (Rodríguez et al. 2005; Tortora et al. 2007).
With the S1 support at both humidities and with S2 and S3 at 20% humidity, it was observed that the loss of viability increased as the storage temperature increased, a negative effect that has been shown by different authors for diverse microorganisms (Chen et al. 2008; Costa et al. 2008; Guijarro et al. 2007; Santos et al. 2012). This effect could be due to the cell metabolism remaining active at a higher temperature, which causes toxic metabolites to be stored that drastically reduce the viability (Santos et al. 2012; Costa et al. 2002). For example, Kinay and Yildiz (2008) evaluated formulations of Pichia guilliermondii and observed that those stored at 4 °C were more stable than those stored at 24 °C. Similar results to those presented in the present study were obtained by Santos et al. (2012), who evaluated the stability of germination of conidia in the Colombian isolations Trichoderma koningiopsis Th003 and Trichoderma asperellum Th034. They were formulated as dry powders for dusting and dispersible granules stored at temperatures of 8, 18 and 28 °C. Said authors obtained longer useful lives when storage was at a temperature of 8 and 18 °C, concluding that the lowest temperatures reduced the metabolic activity of the conidia, preventing the production of toxic metabolites and the exhaustion of nutrients; aspects related to microbial physiology.
The active ingredient mixed with S1 and S2 at 20% humidity were selected as the basic delivery systems for the development of a biopesticide, because they were the most compatible systems with the active ingredient P. fluorescens Ps006, maintaining stable viability and biocontrol activity during three months of storage at 8 ± 2 °C, 18 ± 2 °C and 28 ± 2 °C.
Evaluation of Adjuvants and Packaging Atmosphere
Once the most compatible supports with the active ingredient had been selected, the effect of the presence and absence of oxygen in the packaging and of the two protective adjuvants of the cell membrane (skimmed milk and glycerol) on the viability of P. fluorescens Ps006 in storage conditions was evaluated. The results of said tests are presented in Figure 3.
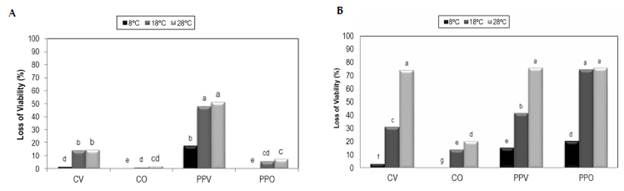
Figure 3 Loss of viability of the active ingredient P. fluorescens Ps006 with the supports A) S1-20% and B) S2-20% after six months of storage. CV: Stored in a vacuum; CO: Stored with oxygen; PPV: Stored in a vacuum with membrane protectors; and PPO: Stored with oxygen with membrane protectors. Treatments with the same letter do not present significant differences according to Tukey's test (95%).
Taking into account the concentration of the cell suspension (1 x 109 CFU/ml) and the proportion of the mixture with the supports, the theoretical concentration of the mixture is estimated to be 1.3 x 108 CFU/ml. The experiment data showed that the initial viability of the active ingredient with the S1 support at 20% humidity was 4.67 x 107 CFU/g, and when it was mixed with the membrane protectors, it was 1.45 x 108 CFU/g. This decrease in the cell concentration when a membrane protector was not used is possibly related to a toxic effect of the support by entering into contact with the P. fluorescens Ps006 cells, which reduced its viability. However, this deleterious effect was decreased by the membrane protectors.
It was observed that the loss of viability after six months of storage was directly proportional to the temperature, a trend that was evident in both atmospheric conditions (with and without oxygen). The treatment without a membrane protector and stored with oxygen was more stable for S1, because the maximum loss of viability was 20% at 28 °C, compared to the other treatments with significantly higher losses (F = 1,622.39; DF = 10; p = 0.0000), which exceeded 70% under the same temperature conditions.
For S2, initial viabilities of 1.79 x 107 CFU/g were obtained when membrane protectors were not used and of 6.90 x 107 CFU/g when they were added, again showing an initial toxic effect upon contact with the solid support. In general, after six months it was observed that the loss of viability increased as the storage temperature increased. Said loss was significantly lower (F = 233; DF = 9; p = 0.0000) for the treatments stored with oxygen compared to the treatments stored in a vacuum. This means that the absence of oxygen in the packaging did not improve the stability of the formulations. This behavior could be due to the stress caused by the vacuum atmosphere on an obligate aerobic bacterium such as P. fluorescens, which generates energy by a process of oxidative phosphorylation with oxygen as its last electron acceptor (Adams & Moss, 2008; Vásquez et al. 2009). The negative effect of the vacuum packaging on the viability of Pseudomonas spp. showed in this study has been widely studied in the food industry, where vacuum packaging is used to inhibit the development of contaminating bacteria (ICMSF, 2006). Preventing the need to implement vacuum systems is an evident advantage for industrial production, because the times and costs of the process are reduced, thus increasing the profitability of the end product.
In most of the cases, the viability was significantly higher (F = 233; DF = 9; p = 0.0000) for the treatments in which the active ingredient was only mixed with the support, compared to the treatments in which, as well as the support, the active ingredient was mixed with the potential membrane protectors. This was with the exception of the S2-20% treatment stored with oxygen, which did not show significant differences when the protectors were used or not. Therefore, glycerol is widely used as a humectant and osmotic protector (Freitas et al. 2009; Rowe et al. 2009), as well as a cryoprotectant and protector against dryness, such as skimmed milk (Cody et al. 2008; Morgan et al. 2006; Barbaree et al. 1982). However, it has been found that certain strains of Pseudomonas spp. use glycerol as a source of carbon to produce biosurfactants (Freitas et al. 2009; Freitas et al. 2010; Silva et al. 2010; Stanghellini & Miller, 1997). This could suggest possible consumption of glycerol and skimmed milk during storage by the formulation prototypes evaluated in the present study. This may have facilitated the growth of the microorganism, maintenance of the cell metabolism and the production of toxic metabolites, causing losses of viability (Santos et al. 2012).
The development of a powder formulation of antagonistic bacteria is especially important for the treatment of seeds (Ramamoorthy et al. 2001). The present study developed a formulation prototype based on the bacterium P. fluorescens Ps006, which survived during six months of storage at 28 °C, having a minimum loss of viability when it was formulated in S1, which is a clay adjusted to 20% humidity, and was used in an uncontrolled packaging atmosphere with air. Similar formulation prototypes have been developed in other studies, but with less stability and in the majority of cases, requiring refrigeration conditions. Such is the case of populations of P. fluorescens formulated in talc with 20% xanthan gum, which maintained their viability during two months of storage at 4 °C (Kloepper & Schroth, 1981). Another formulation that used a vermiculite clay like the present study obtained a stable viability for six months at 4 °C (Connick, 1988). In another study, where the support was talc, the viability was maintained for 90 days at 26 °C (Sadi & Masoud, 2012).
Conclusions
The prototype for formulation in wettable powder (WP) with the active ingredient P. fluorescens Ps006 as a base, which demonstrated its antagonistic activity against the plant pathogens Fusarium oxysporum, Rhizotocnia solani, Botrytis cinerea and Sclerotinia sclerotiorum, was stable for six months of storage at the three evaluated temperatures, when clay adjusted to 20% humidity was used as a support without the addition of membrane protectors and using a packaging atmosphere with oxygen. The losses of viability were less than 5% during the time of storage without refrigeration. Therefore, this prototype was selected to continue with the development of a commercial product with the potential to control diseases in different crops.











 text in
text in