Introduction
There are approximately 1,500 microorganisms that have insecticidal and nematicidal properties, among others (Niedmann and Meza, 2006). Bacillus spp. is considered to be the main agent of biological control or Biocatalytic Effector (EBc®), a versatile pathogen of invertebrates because of its mechanisms of action (Flores et al. 2011; Falconí, 2013a). This kind of bacterium is easily located in places including soil, air, water and plants. (Veitía et al. 2003; Layton et al. 2011). Said microorganisms are considered to be EBc©, because they act through diverse processes and mechanisms in the regulation of individual or collective systems that demonstrate alteration or imbalance (Falconí, 2014a).
According to Expoflores (2013), Tetranychus urticae is a polyphagous mite, commonly known as red spider mite. In ancient times, it was not considered to be a pest of economic importance due to the presence of natural enemies that maintained the balance of said pest. As a result of the indiscriminate use of pesticides since the Second World War, this balance started to disappear. Cerna et al. say that it is currently considered to be one of the species that causes the greatest damage to agriculture worldwide. It is the most abundant pest in roses, causing darkening and weakening in the production of buds, and loss in the quality of the flower, being a recalcitrant problem in rose farming in Ecuador (Rojas et al. 2011; Yánez et al. 2014).
Microorganisms can be identified through different laboratory techniques, for example, the application of Gram staining (Gómez, s/a) and biochemical tests that allow the taxonomic localization of microorganisms to be determined according to their metabolic behavior (García and Silva, 2004). Cytohistochemistry is a process that produces contrasting reactions that translate into pathogenic staining shades in cell components and tissues, which permits the identification of the affected areas of a sample using a microscope (Rodak, 2005; Falconí, 2014b).
As conventional agrochemicals for the control of mites are not efficient, this study aims to isolate and identify strains of Bacillus spp. from T. urticae, and evaluate their cases of pathogenicity compared to a control and to Bacillus thuringiensis biovar acari as a positive control, which has been shown to be efficient in field conditions (Lozada, 2011) as a potential Biocatalytic Effector (EBc®) of T. urticae.
Materials and Methods
Area of Study
The samples were taken from the flower-growing company Naranjo Roses S.A., located at a south latitude of 0°59'46.44" and a west longitude of 78°36'23.52" in Latacunga, province of Cotopaxi. The organic block of roses with the greatest presence of T. urticae and on which no kind of acaricide had been applied was identified. The laboratory phase was carried out in Plantsphere Laboratories (PSL) in Quito, province of Pichincha, where the different samples of rose leaves with T. urticae were processed.
Sampling
In the greenhouse of the Freedom variety of roses, three sources of infection (infected plants) with T. urticae were identified, and in each one of these, 15 leaves were collected from each third (upper, middle and lower). Two samples were taken 15 days apart. The collected leaves were placed in plastic bags without removing the air, aiming to form physical volumes of air to affect the integrity of the biological samples as little as possible. The bags were closed and duly labeled to be transported to the laboratory (adapted from Falconi, 2013b).
Quantification and Identification of Populations of T. Urticae
The methodology used was adapted from Falconi (2013b). Plastic boxes with a lid (6 x 28 x 18 cm) were used, which were carefully disinfected with alcohol (70%). Filter paper with the dimensions of the bottom of the chamber, dampened with sterile distilled water, were placed at the bottom of the boxes. The leaves of the collected roses were placed in each chamber with the lower epidermis facing downward. The chambers were placed in a bright place not directly exposed to sunlight, simulating the natural light conditions in which the pest is located in the field. Each box was duly labeled with the code referring to the source of infection, the third of the leaf it was from, and the date of collection. Each one of the leaves was observed with a microscope at 20X and 40X, proceeding to identify and quantify the different stages of the mites (egg, larva, protonymph, deutonymph, and male or female adult) (Figure 1).
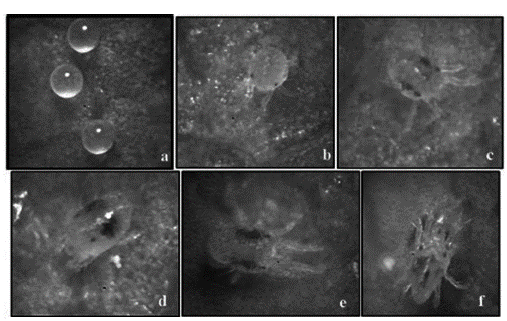
Figure 1 Different stages of T. urticae used in the study: a) Egg; b) Larva; c) Protonymph; d) Deutonymph; e) Female adult; and f) Male adult.
As the count was carried out, the presence of possible pathogens that could be shown in the mites was considered, which are demonstrated in the form of necrosis, tumors, lysis, fluid precipitation and dissolution of tissue.
Eighteen Petri dishes were prepared (for the two sets of samples) with potato dextrose agar (PDA) as a culture medium, in which 20 duly distributed mites were placed. One Petri dish was used for each humid chamber. During the incubation period in the humid chambers in 24-hour cycles, it was considered that if an individual presented some kind of pathology that was duly verified through observation at 20X and 40X, it was isolated, and directly placed in PDA. Since not all the individuals expressed some kind of pathology, four eggs, four larvae, four protonymphs, four deutonymphs and four adults (male and female) were isolated, respectively, at random for each third. Each Petri dish was labeled with the code and date of isolation, and the code to identify the stage was written on the bottom: E (Egg), L (Larva), P (Protonymph), D (Deutonymph), F (Female adult), and M (Male adult). The Petri dishes were incubated at 28 °C, and observed daily for three days to identify any microorganism with characteristics of Bacillus.
Isolation and Identification of Bacillus Spp.
The strains of bacteria were identified as "typical of Bacillus" when they had irregular and flattened borders with a white, matte or shiny color, and a floury, waxy, dry or creamy aspect. Simultaneously, they were isolated in a new Petri dish with PDA for their purification. They were also duly labeled with numbers and PSL codes, incubated at 28 °C, and observed daily for a period of five days to determine that there was no contamination. Subsequently, Gram staining was carried out to determine Gram-positive bacteria using the methodology of Carrea (2009) and four biochemical assays:
a. Catalase test adapted from Zapata (2012).
- Each strain was planted by crossed groove in a nutrient agar culture medium and incubated at 28 °C for 24 hours.
- Three drops of hydrogen peroxide (H2O2) were placed on a coded microscope slide for each strain.
- Using a toothpick, a little of the colony was taken and placed on the first drop. The same was done for the remaining two drops with different toothpicks, thus having three repetitions to test the reaction.
- The test was positive once the formation of bubbles was observed due to the production of oxygen by the enzyme activity.
b. Triple Sugar Iron agar test (Cuervo, 2010)
c. Voges-Proskauer test (Aquiahuatl et al. 2012)
d. Starch hydrolysis test (Carrera, 2009)
Yield of Bacillus Spp. Strains
Under aseptic conditions using the laminar flow cabinet, each strain identified as Bacilllus spp. was planted in PDA by massive inoculation, incubating the strains at 28 °C for four days. In 300 ml glass bottles, 100 ml of phosphate buffer solution were prepared with sterile Tween 80 (10 μl). The PDA in which each strain grew was cut into small squares of approximately 2 cm by 2 cm using a flamed scalpel, and individually placed in bottles with buffer solution. They were shaken in the vortex mixer and then filtered, thus obtaining the base solution of each strain.
Selection of Biocatalytic Effectors©
The first selection of Biocatalytic Effectors (EB©) was carried out in humid chambers (Petri dishes), and a leaf of the Freedom variety rose with T. urticae was placed in them. Three humid chambers (repetitions) were carried out for each of the 15 strains, plus the control (distilled water) and the positive treatment with Bacillus thuringiensis biovar acari. The evaluation was carried out through observation at 20X and 40X, counting the number of individuals and stages to proceed to the inoculation of isolated and generically characterized strains of bacteria. One milliliter of the bacterial solution was placed in test tubes, and using a sprinkler, they were impregnated two times with bacteria in the upper epidermis, as well as the lower epidermis. It was constantly controlled to keep the humid chambers at 50% humidity. After eight days, it was observed in the microscope (20X - 40X) whether the individuals presented some kind of pathogenic anomaly or not, originating from the effect of the metabolic activity of one or more substances involved in the processes of the pest's mortality (Falconi, 2013b).
Cytohistochemistry
The infected leaves were placed on a microscope slide sanitized with 98% alcohol and later treated with three drops of the reactant of the cytohistochemical PSL-CHQ 127. They were left to rest at room temperature for three minutes, and then the individuals of T. urticae were observed at 20X and 40X for three minutes. Three females were identified in each treatment, one for each drop, and evaluated by microscope (1000X). Some alteration in the external morphology of each individual was looked for in each sample, including, for example, dissolution of cell walls, damaged tissue, and necrosis, thus determining the areas that were most affected by the strains of Bacillus spp., compared to the control Petri dishes. The evidence of pathogenic-ity was verified through cytohistochemical techniques, which are used to visualize tissues, systems and organs affected by mechanisms of bacterial control (Falconi, 2013b).
Data Analysis
To determine whether the differences between the mean populations of the groups (upper third (Ut), middle third (Mt) and lower third (Lt)) were significant or not (95% confidence level), a one-way analysis of variance (ANOVA) and the least significant difference (LSD) test for the multiple comparison of means (95% confidence level) were used. The data were processed using the statistical package IBM® SPSS STATISTICS 22.0. The percentage analysis was determined to establish the number of individuals in each third (upper (Ut), middle (Mt) and lower (Lt)). Within this same outline, the average amount of individuals in each third in the sources of infection was determined by stage.
The number of colonies with Bacillus spp. characteristics present in the different T. urticae individuals isolated from the different thirds of the rose leaves was determined. The Gram staining and biochemical tests were determined through positive and negative reaction, thus determining the percentage of strains that produced a positive reaction.
A principal component analysis (PCA) was conducted as a method of synthesis and ordering the treatments that produced the same type or a similar type of pathological effect in adult females of T. urticae. To achieve this, the aforementioned statistical package was used (IBM® SPSS STATISTICS 22.0).
Results and Discussion
Number of Individuals and Identification of Stages of T. Urticae
Table 1 shows the results obtained from the ANOVA (p = 00), which provides evidence that there are differences in the number of individuals between each third. Table 2 shows that the number of individuals of T. urticae decreases as the distance from the basal system increases, because out of the 21,211 counted individuals in the six sources of infection, 59.4% (12,606) was found in the lower third. This is corroborated by Rojas et al. (2011), who localized a greater infestation of the pest in the lower part of the rose plants, due to the higher density of leaves. Furthermore, it has been established that this is a strategy to multiply and protect themselves, because there they are more isolated from the physical and chemical factors of agricultural management of the crop. This indicates that it is a survival mechanism of the pest, which is why it is important to assess the information of the mite control systems (Falconi, 2013b). These results were obtained from two sets of samples, which were taken 15 days apart.
Table 1 ANOVA (Analysis of variance) between the upper thirds (Ut), middle thirds (Mt), and lower thirds (Lt), according to the number of individuals.
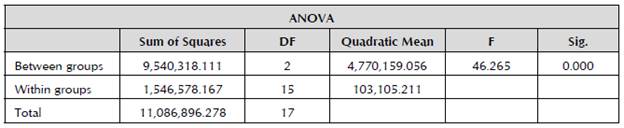
Table 2 Individuals of T. urticae distributed by third in each source of infection in the two samples.
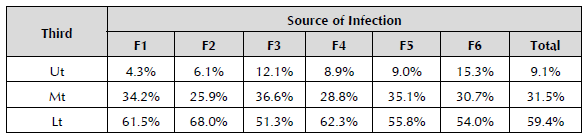
Ut = Upper third; Mt = Middle third; and Lt = Lower third.
Eggs (62.6%) of T. urticae were found in all the thirds, observing that in proportion to the development of the individual until reaching the adult stage, this gradually decreases, as shown in Table 3. This is a result of the sample being taken when the temperature inside the greenhouse fluctuated around 22 °C. The optimum temperature for the development of T. urticae is 25 °C. During their life cycle (20 to 28 days), females lay 100 to 120 eggs. The ratio of adult males to adult females in this study is 6:1. On average, the sex ratio is 3:1 in different crops (Zhang, 2003).
Table 3 Total percentage of the different stages of T. urticae present in each third.
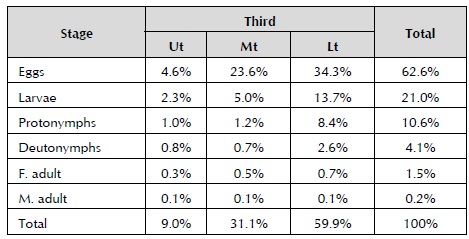
F - Female adult; M - Male adult; Ut - Upper third; Mt - Middle third; and Lt - Lower third.
Table 4 Number of colonies with characteristics of Bacillus spp. present in each source of infection in the different thirds and stages.
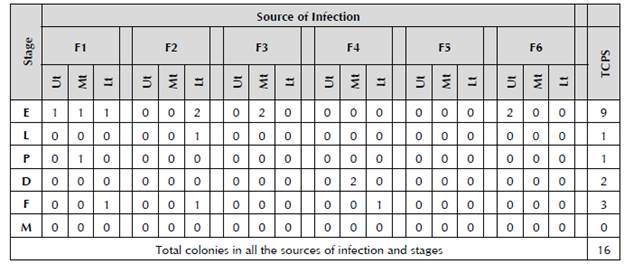
F1 to F6: Sources of infection; Ut: Upper third; Mt: Middle third; Lt: Lower third; TCPS: Total colonies per stage; E: Egg; L: Larva; P: Protonymph; D: Deutonymph; F: Female adult; and M: Male adult.
Presence of Colonies with Characteristics of Bacillus
The highest number of colonies with characteristics of Bacillus spp. was found in the egg stage (9), followed by presence in adult females (3), deutonymphs (2), and in only one individual in the larva and protonymph stages. In total, 16 strains were determined with morphological characteristics of Bacillus spp. (Table 4) with irregular and flattened borders with a white, matte color, and a floury, waxy, dry or creamy aspect like the strains of Bacillus isolated from soil identified in the study by Carrera (2009) and Lozada (2010). These researchers isolated Bacillus subtilis and Bacillus sphaericus from fertilizers with the same characteristics of morphological identification to establish the genus, and later conducted biochemical tests to determine the species. Furthermore, it has been demonstrated in agricultural systems that the numerical presence of the natural regulators has enormous importance for the control of pathogens during some seasons of production (Falconi and Mendgen, 1993; Falconi and Blanco, 2001). Additionally, it could be observed that Bacillus develops in PDA. Therefore, Zhiqiong et al. (2013) conducted tests on the antagonism of Bacillus against Fusarium oxysporum, Alternaría solani, Botrytis cinérea and Colletotrichum gloeosporioides, developing both the bacterium and the fungus in said culture medium to make their respective evaluations. Similarly, in Petri dishes of PDA, Swain and Ray (2006) evaluated the inhibition of in vitro growth of fungi, adding isolated strains of Bacillus subtilis from cow excreta.
Identification of Bacillus Spp.
Out of the 16 strains, PSL 103 was the only Gram-negative bacterium, so it was discarded for conducting the biochemical tests (Table 5), attributable to the isolation of a "pseudomonas type" bacterium, which morphologically presents slightly irregular borders, a mucoid aspect and characteristics of fluorescent pigments (Todar, 2012). All the others were Gram-positive bacteria. In the catalase, starch hydrolysis and Voges-Proskauer biochemical tests, all the strains were positive. In the fermentation of glucose, all of them had a positive reaction, except for Strain 109. Strains PSL 100, 101, 102, 104, 105, 106, 107 and 108 ferment the lactose and/or sucrose, while the other strains do not, and almost all the strains produce gas, except the PSL 113 strain.
Table 5 Positive and negative reaction of the strains of Bacillus spp. in Gram staining and biochemical tests.
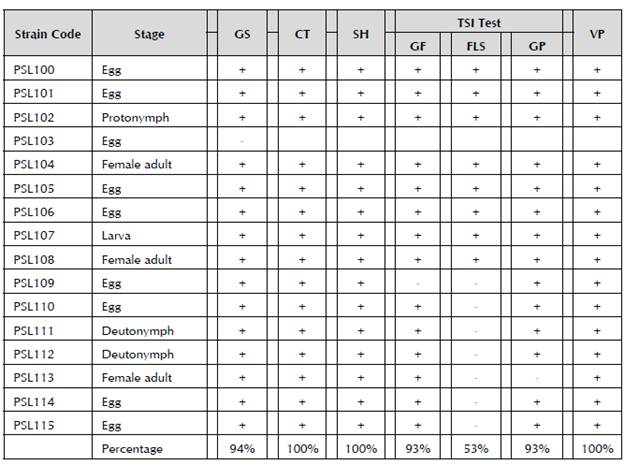
GS (Gram staining); CT (catalase test); SH (starch hydrolysis); GF (glucose fermentation); FLS (fermentation of lactose and/or sucrose); GP (gas production); VP (Voges-Proskauer); + (positive reaction); and - (negative reaction).
Pathogenicity of the Different Treatments
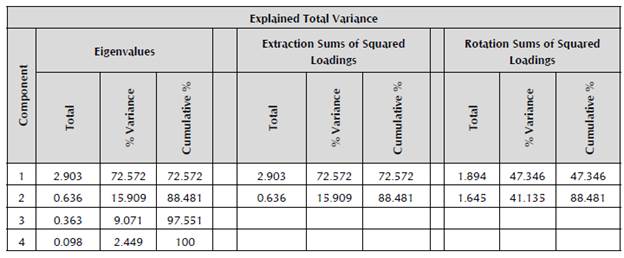
Table 6 shows that Components 1 and 2 accumulate the highest percentage of variance, collectively 88.48% (72.57% and 15.9%, respectively), and Components 3 (9.07%) and 4 (2.45%) were not taken into account for this analysis as their contribution was very low.
Table 6 Extraction of principal component analysis.
Extraction method: Principal component analysis.
Figure 2 shows that the treatments are grouped according to the pathogenic effect on gnathosoma, prodorsum, opistosoma and/or legs in female adults of T. urticae. We can observe that the PSL 104 (Figure 3a), PSL 113, PSL 114 and Bacillus thuringiensis biovar acari treatments are closely related, affecting the four evaluated parts (gnathosoma, prodorsum, opistosoma and legs), which can be applied for tracking in the comprehensive production processes of the crop through molecular techniques (Leibinger et al. 1997). Due to its content of active substances of bacteria, as well as metabolites, the active ingredient Bacillus thuringiensis biovar acari produces a control effect by bioaccumulation, causing the collapse of the nervous, digestive and reproductive systems, drastically reducing the biological activity of the pest (Biocontrol Science, 2009), even in field conditions (Lozada, 2011). PSL 107 (Figure 3c) and PSL 110 are differentiated from the aforementioned treatments, because they affect the gnathoso-ma and prodorsum to a lesser degree. The PSL 105, 115, 109, 100 and 112 treatments are related to the cause of pathogenicity in the opistosoma and legs, while the strains PSL 101, 106 and 108 slightly affect the legs, and PSL 111 highly affects the legs, and affects the opistosoma and prodorsum very little. Finally the PSL 102 and control treatments did not present any kind of pathogenicity in female adults of T. urticae.
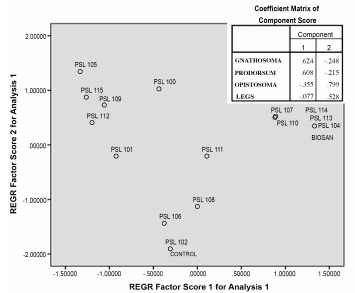
Figure 2 Principal component analysis (PCA) according to the pathogenicity of the different treatments in the gnatho-soma, prodorsum, opistosoma and legs of female adults of T. urticae in Components 1 and 2.
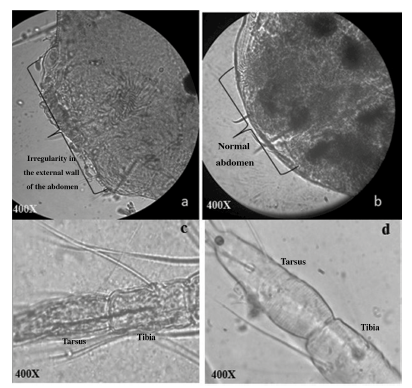
Figure 3 Cytohistochemistry on female T. urticae observed 12 days after applying the inoculation treatment with bacteria. a) Presents irregularity in the external wall of the abdomen caused by the pathogenic activity of the PSL 104 strain. b) Normal external wall of the abdomen. c) Irregularity in the external wall of the cuticle and damaged tissue in the tarsus and tibia of Leg III caused by the PSL 107 strain. d) Leg III without irregularities.
Conclusions and Recommendations
The population effect of T. urticae indicates that the highest number of individuals is located in the lower third, while the sex ratio of T. urticae in roses was six females for each male. Furthermore, a larger amount of eggs was localized (62.6%). Therefore, it is recommended that when applying it in the field, the control must cover the whole area of the plant if possible, because there are always individuals that are far from being affected by the different kinds of control.
The study reports 15 strains of Bacillus spp. through morphological characterization and confirmation by Gram staining and four biochemical tests (catalase test, starch hydrolysis, TSI test and Voges-Proskauer) in which positive results were obtained to determine the Bacillus genus. It is recommended to apply the methodology of endospore staining, because the presence of endospores is another one of the fundamental characteristics of said genus. There are several techniques, and the most used one is the Schaeffer-Fulton technique with the use of malachite green. Additionally, it is recommended to conduct specific biochemical tests to determine the species of Bacillus being studied, or the application of molecular techniques.
As the sampling was carried out in one period when the temperature fluctuated around 22 °C, it is recommended to assess the presence of the bacterial strains detected over extreme weather periods characterized by conditions of deltas affecting plants and organisms in general.
Through the cytohistochemical technique, anatomic, pathogenic scenarios of Bacillus spp. caused in female Tetranychus urticae were evaluated. In these scenarios, rupture of the external walls and precipitation of cell content were observed among others. The PSL 104, 113 and 114 strains were the best, because they acted in the same way as the positive control treatments with Bacillus thuringiensis biovar acari. This indicates the existence of natural regulators in conditions of intensive rose farming, which demonstrate efficiency in invading vital sites of the pest, reducing the damage it causes to the crop. It is important to establish similar studies depending on the search for scenarios of bacterial variability in the Bacillus genus to extend the spectrum of mechanisms of action and modes of action for the control of important agricultural pests, depending on the cycle. It is recommended to conduct field tests of the strains that have been shown to be the most lethal in the laboratory, and determine their effectiveness in biological control programs in flower farm management.
It is also recommended to extract active bacterial ingredients of the most efficient strains as long as one of the main components in the acaricides is comprised of the microbial metabolites of Bacillus thuringiensis biovar acari.











 texto em
texto em 


