Introduction
Sugarcane (Saccharum sp. hybrid) is a plant with a high sucrose content that currently represents one of the world's most important sources of sugar, which is why it is farmed in more than 50 countries. The interest in maintaining and increasing the productivity of this crop is not only sustained by its use in human food, but also by its use in animal feed (Correa et al. 2003; Martín et al. 2005). Specifically in Cuba, work is being carried out on the diversification of the sugar industry, which gives this crop high added value.
Agricultural biotechnology and the use of natural processes play an important role in the implementation of sustainable agriculture. With this objective, plant growth-promoting bacteria (PGPB) are studied, including the bacteria that live in the rhizosphere, as well as rhizobia and endophytic bacteria, which can all benefit the crops (Acemad and Kribet, 2014).
Velázquez et al. (2008) described the genetic diversity of the endophytic bacterial community of apoplastic sap from the medullary parenchyma of sugarcane stems cultivated in Cuban soil. The Gluconacetobacter, Bacillus and Enterobacter genera were isolated from this crop, and the first two were found to be the most abundant in the bacterial community.
Studying the effect of different sources of carbon and nitrogen related to the natural environment, as well as other components of it, will allow to explain the endophytic microorganisms' behavior in their natural habitat. This will allow more in-depth knowledge of the plant-bacterium interaction and support for the bacterium's use in agricultural biotechnology. Additionally, this study lays the foundations for the formulation of efficient culture media based on natural sources for the endophytes' growth.
This work aims to demonstrate the effect of different sources of carbon and nitrogen on the growth of five endophytic strains found in sugarcane. Additionally, the influence of juices from the different varieties on the growth of these bacteria was studied, as well as different concentrations of the plant hormones indole-3-acetic acid (IAA) and gibberellic acid (GA).
Materials and Methods
Strains Used
The following bacterial strains were used: Gluconacetobacter diazotrophicus PAl5 (ATCC 49037), a strain isolated from roots of sugarcane in Brazil (Cavalcante and Dobereiner, 1988); G. diazotrophicus 1-05 and 4-02 strains isolated from the stems of the Jaronú 60-5 and Media Luna 318 sugarcane cultivars, respectively (Rojas, 2005); Bacillus licheniformis isolated from the stems of the Cuba 323-68 sugarcane variety (Rojas et al. 2001) and Enterobacter agglomerans isolated from the stems of the Media Luna 318 sugarcane variety (Rojas et al. 2001).
Influence of the Source of Carbon and Nitrogen on Growth
The strains were cultivated in liquid LGI medium (in g/l, 0.2 K2HPO4; 0.6 KH2PO4; 0.2 MgSO4 7H2O; 0.02 CACL2 2H2O; 0.02 NaMoO4 2H2O; 0.01 FeCL3 6H2O; 5 ml of bromothymol blue (0.05% solution in 0.2 N KOH)) with 10 g/l of sucrose as a source of carbon (Cavalcante and Dobereiner, 1988) for 48 hours at 30 °C and 150 rpm. The inoculums were adjusted to 108 UFC/ml, according to the McFarland standard (CLSI, 2005) with 0.8% saline solution (0.8 g NaCl in 100 ml of H2O). A semisolid LGI medium was prepared (2 g/l of agar) with the following sources of carbon in a final concentration of 1%: xylose, sucrose, inositol, glucose, fructose and sugarcane juice.
Culture media were prepared with 1% sucrose as a source of carbon and the following sources of nitrogen in a final concentration of 5 mM: ammonium sulfate, potassium nitrate, asparagine, tryptophan, threonine, arginine and a control without the addition of nitrogen.
In every 5 ml of medium, 100 μl of inoculum were planted and incubated for 72 hours at 30 °C. The absorbency was measured at 600 nm. Everything was determined three times.
Influence of Juices of Different Sugarcane Varieties on Growth
An inoculum similar to the one described in the summary above was prepared, and it was also adjusted to 108 UFC/ml. From this preparation, 100 μl were taken to inoculate tubes with cultures that contained 5 ml of liquid LGI medium and sugarcane juice from different sugarcane cultivars for a final concentration of 20 and 30%, as well as a control with commercial sugar as a source of carbon. The juice of five different sugarcane cultivars was used. The samples were incubated at 150 rpm, and 30 °C, and the absorbency was measured at 600 nm after 24, 48 and 72 hours. Everything was determined three times.
Influence of Indole-3-acetic Acid and Gibberellic Acid on Growth
A similar procedure was used to the one described in the summary above, using the same SYP (in g/l, 10 sucrose, 3 yeast extract, 1 K2HPO4, 3 KH2PO4, pH = 6.2) (Caballero-Mellado and Martínez-Romero, 1994) liquid medium with different final concentrations of indole-3-acetic acid (IAA) and gibberellic acid (GA) of 0.025, 0.25 and 2.5 mg/l, and the medium without hormones as a control. The samples were incubated at 150 rpm and 30 °C, and the absorbency was measured at 600 nm after 24, 48 and 72 hours. Everything was determined three times.
Biometric Analysis
The results of the described physiological studies were analyzed using the Tonystat (9) statistical package. Parametric and non-parametric tests were conducted: analysis of variance, Kruskal-Wallis test, Duncan's new multiple range test and non-parametric multiple comparison test.
Results and Discussion
Influence of the Source of Carbon and Nitrogen on Growth
In the studied strains, significant differences can be observed in growth between the media with different sources of nitrogen (Table 1). The media with ammonium sulfate and asparagine are highlighted as sources of nitrogen for the strains of G. diazotrophicus. For B. licheniformis, the media with ammonium sulfate, potassium nitrate, asparagine and arginine show the highest growth values. The last two sources of nitrogen are repeated for E. agglomerans.
Table 1 Growth of strains of sugarcane endophytes in LGI medium with different sources of nitrogen measured in cultures of 5 ml through absorbency at 600 nm.
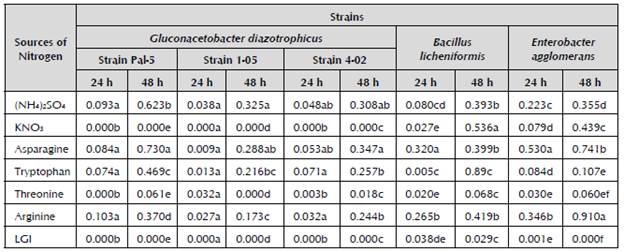
Different letters indicate significant differences (p ≤ 0.05) using Duncan's new multiple range test. Mean of three repetitions.
Stephan et al. (1991) proved the action of several amino acids on the nitrogenase activity of G. diazotrophicus.Rodríguez et al. (2005) demonstrated that with threonine, tryptophan, arginine and asparagine as sources of nitrogen, there is evidence of partial inhibition of this activity. This is perhaps a result of similar deactivation mechanisms of the biological fixing of nitrogen, which are possibly not so efficient, which is consistent with the results obtained in growth inhibition in this study.
This bacterium has shown its promoting effect on the growth of different crops of economic importance such as Colocasia esculenta, cassava and papaya. Field studies have demonstrated its effect on increasing the yields and quality of the fruits or tubers (Dibut et al. 2009).
After 24 hours of incubation in media with different sources of carbon, there are no significant differences in the growth of the strains of G. diazotrophicus (Table 2). However, for B. licheniformis and E. agglomerans, differences in growth are observed from 24 hours. This was to be expected, because these bacteria had a higher growth rate than G. diazotrophicus.
Table 2 Growth of sugarcane endophytes in LGI medium with different sources of carbon measured in 5 ml cultures through absorbency at 600 nm.
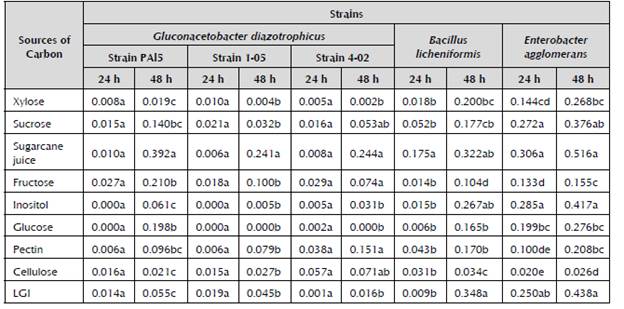
Mean of three repetitions. Different letters indicate significant differences (p ≤ 0.05) using Duncan's new multiple range test.
The results obtained with the strains of G. diazotrophicus are consistent with those presented by Ureta et al. (1995). These authors observed that there are differences between strains of the same species in terms of the use of sources of carbon. In this research, differences in growth were observed in the media with xylose, fructose, inositol, glucose and pectin. When identifying 46 isolated of this species from several cultivars of sugarcane, Rojas et al. (2012) demonstrated that there are strains that do not grow in the media with glycerol, mannitol, xylose and inositol as sources of carbon, which is consistent with our results. This work demonstrates that there are different behaviors in the growth for the studied strains after being analyzed quantitatively, even when the strains grow in the analyzed media. This is important for the future use of these bacteria in agricultural biotechnology. As demonstrated by Dibut et al. (2011), the best results are obtained by sprinkling the leaves and the soil of the G. diazotrophicus crop. Therefore, the use of a wide range of sources of carbon could sustain the bacterium's survival.
Through poteomic studies, Lery et al. (2008) identified numerous proteins involved in the metabolism of amino acids, carbohydrates and others in the Pal5 strain, which supports the use of the compounds analyzed in this study. Even Luna and Boiardi (2007) demonstrated that the use of glucose depends on the medium's pH.
Using RT-qPCR, Galisa et al. (2012) demonstrated the differentiated expression of genes of maintenance and others related to biosynthesis and catabolism in LGI medium supplemented with different sources of carbon. This corroborates the differences in growth found in this research.
The difference between the medium that has sugarcane juice as a source of carbon and the other sources of carbon used is observed after 48 hours of growth for G. diazotrophicus and from 24 hours for B. licheniformis and E. agglomerans. The elevated growth in LGI medium with sugarcane juice could be due to the presence of vitamins, proteins and other compounds that facilitate the growth of the microorganisms. Taking into account the results obtained in the LGI medium with sugarcane juice, and that there are differences in the composition of the juice of the sugarcane cultivars, it was decided to test whether the juice's variety of origin had an influence on the growth of this species. This is due to the importance this could have when formulating a culture medium for the biotechnological application of these bacteria in agriculture.
Influence of Juices of Different Sugarcane Cultivars on Growth
After 24 and 48 hours of culture in the LGI medium supplemented with juice of different varieties of sugarcane at concentrations of 20 and 30%, there are no differences in the growth of the analyzed strains.
After 72 hours, in the case of the 4-02 strain of G. diazotrophicus, growth is still significantly higher in the medium without juice using commercial sugar at 20 and 30% (Figure 1). This could suggest that the compounds present in sugarcane juice at certain concentrations stimulate microbial growth, but that from a determined concentration, toxic substances may be produced that inhibit the growth of some strains.
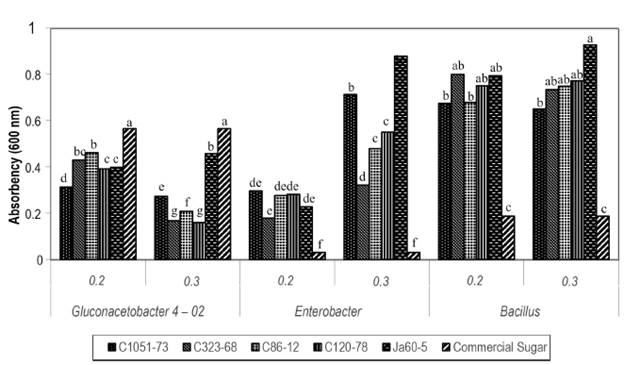
Figure 1 Growth of endophytic strains of sugarcane in juices of different cultivars (C1051-73, C323-68, C86-12 and Ja605) added at 20 and 30% to the LGI medium after 72 hours of culture, shaken at 150 rpm and 30 °C. Different letters indicate significant differences within the same bacterial strain using Duncan's new multiple range test (p ≤ 0.05).
After 72 hours of growth in all the media with juice, Enterobacter agglomerans and B. subtilis exceeded the LGI medium with commercial sugar in growth. In this case, it could be considered that sugarcane juice has a positive influence on the growth of the bacteria, because the juice contains glucose and fructose (0.2-0.6%), organic nitrogen (0.02-0.04%) and vitamins (Reis et al. 1994). Furthermore, the poor growth of these microorganisms in the medium with commercial sugar compared to the growth in sugarcane juice could indicate the presence of industrial waste from the sugarcane industry that harms the bacteria's growth.
The difference in growth is notable in the media with juice from the cultivars C1051-73 and Ja605 at 30% for the strain of E. agglomerans, which have the indicator of total soluble solids (Brix) and the pol percentage (pol: mainly formed of sucrose) in common (Naranjo, S.).
The presence of large amounts of ions such as K+ and organic acids (Wealbun et al. 1990) among the soluble solids could stimulate the growth of E. agglomerans, but it is more reasonable to believe that the pol percentage has a bigger influence. This essentially takes into account the percentage of sucrose, but carbohydrates of medium molecular weight could also be present, which are optically active substances, and it has been demonstrated that they divert the plane of polarized light and could influence the measurements of sucrose by polarimetry (De Armas et al. 1999). In turn, these carbohydrates could be used by the microorganism, because they are polymers of fructose and galactitol (Legaz et al. 1992). It is worth clarifying that no literature has been found on researches that compare the growth of bacteria in different sugarcane juices, and that these studies could lay the foundations for the formulation of culture media with cheaper sources for the agricultural biotechnological application of plant growth-promoting bacteria.
Influence of Indole-3-acetic Acid and Gibberellic Acid on Growth
There are very few studies that analyze endophyte-plant interrelationship with respect to the plant hormones' influence on the bacteria's physiology.
For E. agglomerans, it is observed that concentrations of 0.025 and 0.25 mg/l of both plant hormones stimulate growth compared to the SYP medium without hormones after 72 hours (Figure 2). Therefore, it can be inferred that induction of growth occurs at these concentrations of IAA and GA, as it occurs with Azospirillum brasilense in the presence of IAA. This is explained by stimulated regulation of the genes that codify for the indole-3-pyruvate decarboxylase enzyme, which is involved in the pathway of IAA synthesis by the microorganism (Vande Broek et al. 1999).
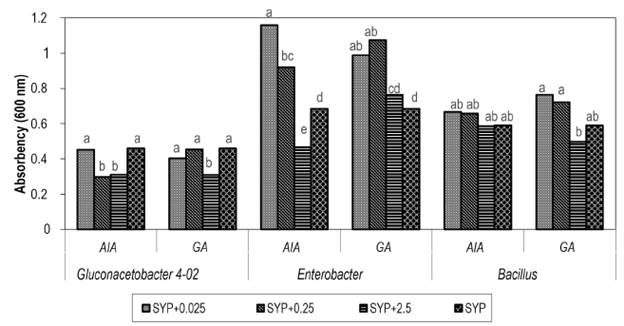
Figure 2 Influence of indole-3-acetic acid (IAA) and gibberellic acid (GA) on the growth of sugarcane endophytes after 72 hours of culture in SYP medium supplemented with different concentrations of hormones (mg/l). Different letters indicate significant differences within the same bacterial strain using Duncan's new multiple range test (p ≤ 0.05).
In turn, the IAA in the plant stimulates protein synthesis because it stabilizes the messenger RNA, which increases the rate of transcription, and the GA3 empowers the presence of polyribosomes and increases the number of ribosomes per cell (De Armas et al. 1988), which may exercise a similar action over the microbial cell.
Conclusions
It may be posed that the use of asparagine and ammonium sulfate as sources of nitrogen added to the LGI medium facilitates greater growth of the endophytic bacteria in sugarcane.
The LGI medium supplemented with sugarcane juice significantly facilitates the growth of endophytic microorganisms and there is no direct relationship between the juice's variety of origin and the bacteria's growth. Therefore, it could be used in a culture medium for the biotechnological production of the endophytes.
The study of the ecology on the interaction between the endophytes and the plants is of great importance. More specifically, the study of the relations of the plant's components, whether the juice or the plant hormones, could be extremely interesting and open doors to analyze the in vivo plant-endophyte interaction in future work.











 text in
text in 


