Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Biotecnología
Print version ISSN 0123-3475
Rev. colomb. biotecnol vol.18 no.1 Bogotá Jan./June 2016
https://doi.org/10.15446/rev.colomb.biote.v18n1.57724
DOI: http://dx.doi.org/10.15446/rev.colomb.biote.v18n1.57724
ARTÍCULO DE REVISIÓN
Procedimientos actuales para la extracción y purificación de flavonoides cítricos
Current procedures for extraction and purification of citrus flavonoides
Andrés Alejandro Damián-Reyna*, Juan Carlos González-Hernández**, Ma. del Carmen Chávez-Parga*,***
* Facultad de Ingeniería Química, Universidad Michoacana de San Nicolás de Hidalgo, Ciudad Universitaria, Francisco J. Mújica S/N, Col. Felicitas del Río, 58030, Morelia, Michoacán, México. Correo electrónico: pandamian77@hotmail.com.
** Laboratorio de Bioquímica del Departamento de Ing. Bioquímica del Instituto Tecnológico de Morelia, Av. Tecnológico # 1500, Colonia Lomas de Santiaguito, 58120, Morelia, Michoacán, México. Correo electrónico: jcgh1974@yahoo.com.
*** Correo electrónico: cparga@umich.mx
Recibido: agosto 10 de 2015 Aprobado: abril 25 de 2016
Resumen
En la industria alimenticia, los agentes microbicidas son usados para preservar la calidad y seguridad de los alimentos procesados. Los flavonoides encontrados en extractos cítricos han mostrado capacidad de inhibición del crecimiento celular de un gran grupo de microorganismos infecciosos, por lo tanto, éstos compuestos pueden ser útiles como agentes antivirales, antifúngicos y antibacteriales. Los flavonoides que se pueden encontrar principalmente en varias de las especies cítricas son hesperetina, hesperidina, luteolina, naringenina, naringina, narirutina, neohesperidina, nobiletina y tangeretina. A continuación se resumen los procesos utilizados recientemente para extraer, purificar y analizar los flavonoides principales en frutas cítricas.
Ya optimizado el medio de cultivo se procedió a realizar una cinética confirmatoria con base a las condiciones encontradas pero evaluando el crecimiento celular por conteo en cámara de Neubauer y la producción de etanol mediante método enzimático (Procedimiento de ensayo K-ETOH, Megazyme) para confirmar los valores.
Palabras clave: flavonoides, polifenoles, purificación, cítricos, microbicida.
Abstract
In the food industry, antimicrobial agents are used for preserving the quality and safety of processed food. Flavonoids found in citrus extracts inhibit cell growth of a large group of infectious microorganisms, therefore, these compounds may be useful as antiviral, antifungal and antibacterial agents. Hesperetin, hesperidin, luteolin, naringenin, naringin, narirutin, neohesperidin, nobiletin and tangeretin are some of the main flavonoids found in various citrus fruits. The processes used in recent years to extract, purify and analyze typical flavonoids from citrus species are reviewed.
Key words: flavonoids, polyphenol, purification, citrus, antimicrobial.
Introduction
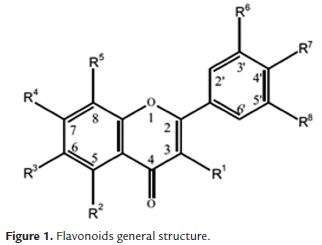
Flavonoids are a group of polyphenols that are found in fruit, vegetables, nuts, seeds, stems and flowers as well as tea, wine, propolis and honey (Tham & Liew, 2014). Some flavonoids are responsible for fruit coloration. Most of flavonoids are structured basically by 3 rings, two of them are aromatic benzene rings (called rings A and B), connected by an oxygen pyrane ring (called ring C), as shown in figure 1, and there is the characteristic presence of hydroxyl groups in one or more R positions.
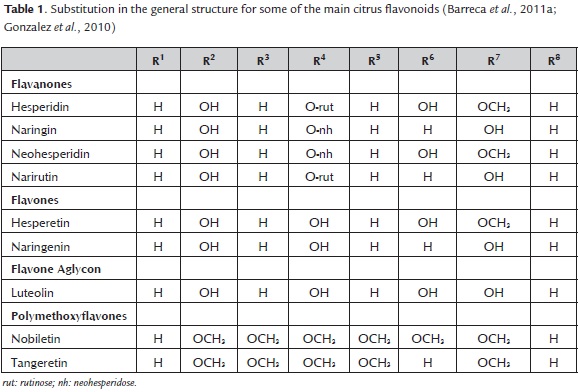
Especially abundant in citrus fruits, there are some flavonoids that can be found in almost all citrus fruits (table 1), like hesperidin, which consolidate a group called citrus flavonoids. Their concentration in peels is higher than in juice and seeds (Codoñer & Valls, 2010) as a result of flavonoids role for fruit coloration.
Citrus flavonoids are well known for their antioxidant (Asikin et al., 2015; Yu et al., 2014; Barreca et al., 2011a; Barreca et al., 2011b; Pekal et al., 2011; Procházková et al., 2011; Ye et al., 2011; Kelebek, 2010) antifungal (Buer et al., 2010; Montes, 2009), and antimicrobial effect (Celiz & Audisio, 2011; Cushnie & Lamb, 2011; Vikram et al., 2010), and even for accelerating wound and disease healing (Wang et al., 2014; Arab & Liebeskind, 2010; Codoñer & Valls, 2010; Neves et al., 2010).
For nourishment purposes, research has concluded that consumers in theory are ready to accept food rich in flavonoids, by informing them about the scientific benefits (Zang et al., 2014; Zhang et al., 2014a; Jung et al., 2011; Lampila et al., 2009). In Europe, an average adult person spends up to €454.7 per year in flavonoids contained in cardiovascular drugs (Sanfelix et al., 2010).
Raw materials
Citrus fruits contain a range of key nutrients such as vitamin C, folate, dietary fiber, minerals and phytochemicals, which attributes to their health-promoting properties (Ledesma & Luque, 2014). It is believed that vitamin C is a major contributor to the anti-oxidant capacity of citrus. However, the major contribution of citrus anti-oxidant activity comes from the combination of phytochemicals and from their synergistic action with vitamin C. The major phytochemicals in citrus fruits are the terpenes and phenolic compounds, which possess anti-inflammatory and anti-carcinogenic activity (Wang et al., 2014; Natarajan et al., 2011; Codoñer & Valls, 2010).
The main citrus fruits in flavonoids research have been orange (C. sinensis), lemon (C. limon), grapefruit (C. paradisi) and tangerine (C. reticulata), for at least the past 30 years. Nowadays, native varieties are of special interest. Tough Citrus species are harvested all around the world (Lorente et al., 2014), some species are better developed than others, depending on climate conditions, and its availability varies from one country to another or even regions within the same country (Roussos, 2011). This influences the research on specific Citrus species (table 2), where the main citrus producing countries have more studies on a wider variety of species, exploring even the wild varieties found in unique locations.
Raw material conditioning
Citrus have been collected and extracted in early springtime or late winter (Sandoval et al., 2012; Barreca et al., 2010), and about 2-5 months after the flowering period (Barreca et al., 2011a; Chen et al., 2011; Yoo et al., 2009). Even though most compounds are found in the peel, as indicated before, studies also have been made to obtain extracts rich in flavonoids from the juice and the seeds. In order to facilitate the extraction of the components, raw material must be ground to a small particle size, improving extract transport from the solid matrix towards the solvent phase.
Raw material can be used either fresh or dry (Ye et al., 2011), the use of fresh raw materials involves the presence of an aqueous phase in the extract, and a further separation, like decantation, must be carried out.
When dry raw material is used, it must be conditioned first, in order to permit cells to stretch back to their original size and shape, allowing the components to transfer through the cell's structure into solvent bulk. Juices, peels and other tissues can be separated manually (Barreca et al., 2011a) or using commercial extractors.
Preliminary Separation
Organic solvents are often used for the extraction of citrus compounds. There are two main operations to extract the compounds from the citrus matrix. One is simple maceration, with solvents extracting the compounds by diffusion from the citrus matrix (Yoo et al., 2009), where methanol is a frequently used solvent (table 3). The second one is centrifugation of the juices, eliminating aqueous phases (Barreca et al., 2011a).
Purification
The mixtures obtained from extraction are quite complex, showing many species from the different tissues in the fruit. In order to obtain a higher concentration of some compounds, it's necessary to carry out a further purification.
Compound purification has been carried out by column chromatography, allowing high single concentration of compounds (Levaj et al., 2008). Purification through adsorption is versatile, simple and low cost, which makes it attractive for the selective recovery of a variety of phenolics and polyphenols. Adsorption shows other advantages like selectivity, environmental impact and toxicological effects. Studies on the characterization of the detailed interactions between resins and individual plant phenolics are needed for design (Soto et al., 2011).
High speed countercurrent chromatography (HSCC) is also used to extract and purify flavonoids using two-phase solvent systems, flowing simultaneously in opposite directions. In addition, HSCC also realizes multiple forms of the gradient elution process; thus it can be used not only to remove impurities from crude extract of raw materials but also to purify the final product. Moreover, some pure compounds can even be obtained through one step from crude extract without sample pretreatment (Duo et al., 2011).
Even tough, there is few available data for citrus flavonoids purification processes, i.e. the one used for purifying hesperidin, naringin, and narirutin with a Zorbax C18 column and a mobile phase of citric acid and ammonium acetate in water and methanol 60:40 (Levaj et al., 2008). Also, preparative high performance liquid chromatography (HPLC) using an instrument equipped with a UV-vis detector has not been employed widely in the isolation of flavonoid compounds. Most mobile phases consisted of a linear gradient of acetonitrile in H2O. Crude juice is diluted with DMF, flavonoids are collected in the HPLC course range time of 5-30 min. The fractions collected are joined, evaporated to dryness in a rotary evaporator and redissolved to regenerate the original concentration of analytes in crude juice (Barreca et al., 2011d).
Analysis methods
Once the extract is obtained, it's important to analyze it, to know if the procedure was correct and the present species were separated as expected.
Qualitative methods
Thin layer chromatography (TLC) continues to be an important method for qualitative investigation of plant compounds because of its inherent advantages- many samples can be analyzed simultaneously and quickly and multiple separation techniques and detection procedures can be applied. TLC is one of the most powerful and general analytical tools used for qualitative purposes, indicating the presence of specific flavonoids in a simpler way than HPLC. It follows from numerous publications that, owing to large polarity differences, it is difficult to find a TLC system which separates similar structure molecules on a single chromatogram.
Most common stationary phase is silica gel, using a mobile phase of mixtures ethyl acetate - methanol - formic acid (Mohammad et al., 2010). Conventional separation on silica gel with moderately polar mobile phases consisting of small amounts of methanol with less polar solvents has been successfully used for the polyphenolic compounds. The retention factor (Rf) values of the different compounds reflects their polarity, given by the number of -OH groups, wich displays much more affinity for the stationary phase.
Using the Folin-Ciocalteu reaction, the phenolic compounds form blue complexes with the phosphomolybdic- phosphotungstic reagent at high pH. The analysis is simple, highly reproducible under carefully controlled conditions, and, therefore, widely used. The Folin method represents a classic approach to estimate total phenolic compounds in a variety of matrices. Although the method is nonspecific, it is frequently applied as a measure of total phenolics in biochemical, animal, and clinical trials (Soto et al., 2012). Fluorescence detection of the flavonoids is also used to identify the effective separation of individual flavonoid compounds (Andreu et al., 2010).
Quantitative methods
High performance liquid chromatography (HPLC) is widely used to quantify the amount of flavonoid compounds in the obtained extracts, and there are several methods reported for HPLC sets (table 4). For every method, it must be considered the polarity of the species to be analyzed, so the correct column and mobile phase may be chosen. The hypothesis proposes that the difference in the orientation of the -OH could result in different affinities of the two isomers for the stationary phase and hence their separation. Good characterization of mobile-phase systems can be achieved by determination of relationships between retention and mobile-phase composition.
Gas chromatography is also used, but due to its characteristics, volatile samples are required (Cheong et al., 2012). In addition, water samples are not allowed, only the species that are soluble in volatile solvent should be measured.
Liquid chromatography has been the most used technique to analyze the obtained extracts from citrus fruits (Jiang et al., 2011), performing tests at different pH levels and using a huge variety of detection methods. High performance liquid chromatography (HPLC) combined with ultraviolet (UV) detection and mass spectrometric (MS), electrospray ionization (ESI), and/or two mass spectrometer tandem (MS/MS) measurement provides the most useful techniques currently available to identify specific classes and structures of food phenolics (Barreca et al., 2013; He et al., 2011). The differences in ultraviolet spectra are an important tool in determining which wavelengths to monitor for detection and quantification by HPLC (Soto et al., 2012; González et al., 2010).
Conclusions
Since citrus fruits are original from Asia, most of the varieties on the current literature were found and studied in Far East countries. It doesn't mean that others countries are not interested in studying citrus flavonoids, only that they don't have so much of wild or endemic citrus species. Most of the studies used grounded dry raw material for extraction, from peels and whole fruit. Methanol mixtures are the main solvent used in the extraction of citrus flavonoids.
There's few literature found about purification of single flavonoids, since few details of purification behavior of single flavonoid compounds have been provided in most of the publications dealing with their isolation and structural elucidation, and, in some cases, inadequate information is supplied, there is an entire opportunity field for new research in purification techniques, and their efficiencies in flavonoids isolation.
High performance liquid chromatography has been used as the best analysis technique to quantify and identify structures of the obtained flavonoids and thin layer chromatography provides a quick method for qualitative identification of the compounds along the experimental process.
Acknowledgements
Authors are grateful to Postgraduate Department of Chemical Engineering at Universidad Michoacana de San Nicolás de Hidalgo for providing access to databases. The work was supported by the CONACYT, México, scholarship number 220045/206495.
References
Abad-García, B., Garmón-Lobato, S., Sánchez-Ilárduya, M.B., Berrueta, L.A., Gallo, B., Vicente, F., & Alonso-Salces, R.M. (2014). Polyphenolic contents in citrus fruit juices: authenticity assessment. European Food Research and Technology, 238, 803-818. [ Links ]
Andreu-Navarro, A., Fernandez-Romero, J.M., & Gómez-Hens, A. (2010). Long-Wavelength Fluorescence Detection of flavonoids in orange juices by LC. Chromatographia, 72, 1115-1120. [ Links ]
Alvarez, R., Carvalho, C.P., Sierra, J., Lara, O., Cardona, D., Londoño, J. (2012). Citrus juice extraction systems: Effect on chemical composition and antioxidant a Activity of clementine juice. Journal of Agricultural and Food Chemistry, 60, 774-781. [ Links ]
Arab, L. & Liebeskind, D. (2010). Tea, flavonoids and stroke in man and mouse. Archives of Biochemistry and Biophysics, 501, 31-36. [ Links ]
Asikin, Y., Maeda, G., Tamaki, H., Mizu, M., Oku, H., & Wada, K. (2015). Cultivation line and fruit ripening discriminations of Shiikuwasha (Citrus depressa Hayata) peel oils using aroma compositional, electronic nose, and antioxidant analyses. Food Research International, 67, 102-110. [ Links ]
Barreca, D., Bellocco E., Caristi C., Leuzzi U., & Gattuso G. (2010). Flavonoid composition and antioxidant activity of juices from chinotto (Citrus x myrtifolia Raf.) fruits at different ripening stages. Journal of Agricultural and food Chemistry, 58, 3031-3036. [ Links ]
Barreca D., Bellocco E., Caristi C., Leuzzi U., & Gattuso G. (2011a). Distribution of C- and O-glycosyl flavonoids, (3-hydroxy-3-methylglutaryl) glycosyl flavanones and furocoumarins in Citrus aurantium L. juice. Food Chemistry, 124, 576-582. [ Links ]
Barreca D., Bellocco E., Caristi C., Leuzzi U., & Gattuso G. (2011b). Elucidation of the flavonoid and furocoumarin composition and radical-scavenging activity of green and ripe chinotto (Citrus myrtifolia Raf.) fruit tissues, leaves and seeds. Food Chemistry, 129, 1504-1512. [ Links ]
Barreca D., Bellocco, E., Caristi, C., Leuzzi, U., & Gattuso, G. (2011c). Flavonoid profile and radical-scavenging activity of Mediterranean sweet lemon (Citrus limetta Risso) juice. Food Chemistry, 44, 2190-2197. [ Links ]
Barreca, D., Bellocco, E., Caristi, C., Leuzzi, U., & Gattuso, G. (2011d). Kumquat (Fortunella japonica Swingle) juice: Flavonoid distribution and antioxidant properties. Food Chemistry, 129, 417-422. [ Links ]
Barreca, D., Bellocco, E., Leuzzi, U., & Gattuso, G. (2014). First evidence of C- and O-glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties. Food Chemistry, 149, 244-252. [ Links ]
Barreca, D., Bisignano, C., Ginestra, G., Bisignano, G., Bellocco, E., Leuzzi, U., & Gattuso, G. (2013). Polymethoxylated, C- and O-glycosyl flavonoids in tangelo (Citrus reticulata x Citrus paradisi) juice and their influence on antioxidant properties. Food Chemistry, 141, 1481-1488. [ Links ]
Brito, A., Ramirez, J.E., Areche, C., Sepúlveda, B., & Simirgiotis, M.J. (2014). HPLC-UV-MS Profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules, 19, 17400-17421. [ Links ]
Buer, C.S., Imin, N., Djordjevic, M.A. (2010). Flavonoids: New roles for old molecules, Journal of Integrative Plant Biology, 52(1), 98-111. [ Links ]
Celiz, G., Daz, M. & Audisio, M.C. (2011). Antibacterial activity of naringin derivatives against pathogenic strains. Journal of Applied Microbiology, 111, 731-738. [ Links ]
Chen, M.L., Yang, D.J., & Liu, S.C. (2011). Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. International Journal of Food Science and Technology, 46, 1179-1185. [ Links ]
Cheong, M.W., Zhu, D., Sng, J., Liu, S.Q., Zhou, W., Curran, P., Yu, B. (2012). Characterization of calamansi (Citrus microcarpa). Part II: Volatiles, physicochemical properties and non-volatiles in the juice. Food chemistry, 134, 696-703. [ Links ]
Codoñer-Franch, P. & Valls-Bellés, V. (2010). Citrus as functional foods. Current Topics in Nutraceutical Research, 8(4), 173-184. [ Links ]
Costa, R., Russo, M., DeGrazia, S., Grasso, E., Dugo, P., & Mondello, L. (2014). Thorough investigation of the oxygen heterocyclic fraction of lime (Citrus aurantifolia (Christm.) Swingle) juice. Journal of Separation Science, 37, 792-797. [ Links ]
Cushnie, T.P.T. & Lamb, A.J. (2011). Recent advances in understanding the antibacterial properties of flavonoids. International Journal of Antimicrobial Agents, 38, 99- 107. [ Links ]
DiDonna, L., Galluci, G., Malaj, N., Romano, E., Tagarelli, A., Sindona, G. (2011). Recycling of industrial essential oil waste: Brutieridin and Melitidin, two anticholesterolaemic active principles from bergamot albedo. Food chemistry, 125, 438-441. [ Links ]
DiDonna, L., Taverna, D., Mazzoti, F., Benabdelkamel, H., Attya M., & Sindona G. (2013). Comprehensive assay of flavanones in citrus juices and beverages by UHPLC-ESI-MS/MS and derivatization chemistry. Food chemistry, 141, 2328-2333. [ Links ]
Duan, L., Guo, L., Dou, L.L., Yu, K.Y., Liu, E.H., & Li, P. (2014). Comparison of chemical profiling and antioxidant activities of fruits, leaves, branches, and flowers of Citrus grandis 'Tomentosa'. Journal of Agricultural and Food Chemistry, 62, 11122-11129. [ Links ]
Duo-Long, D. Yuan-Yuan, Z., Xiao-Fen, C., Xin-Yi, H, & Shi-Lan, F. (2011). Advances in application of high-speed countercurrent chromatography in separation and purification of flavonoids. Chinese Journal of Analytical Chemistry, 39(2), 269-275. [ Links ]
González-Molina, E., Domínguez-Perles, R., Moreno, D.A., & García-Viguera, C. (2010). Natural bioactive compounds of Citrus limon for food and health. Journal of Pharmaceutical and Biomedical Analysis, 51, 327-345. [ Links ]
Guimaraes, R., Barros, L., Barreira, J.C.M., Sousa, M.J., Carvalho, A.M., Ferreira, I.C.F.R. (2010). Targeting excessive free radicals with peels and juices of citrus fruits: Grapefruit, lemon, lime and orange. Food and Chemical Toxicology, 48, 99-106. [ Links ]
Hamdan, D., El-Readi, M.Z., Tahrani, A., Herrmann, F., Kaufmann, D., Farrag, N., El-Shazly, A., Wink, M. (2010). Chemical composition and biological activity of Citrus jambhiri Lush. Industrial Crops and Products, 32, 269-274. [ Links ]
He, D., Shan, Y., Wu, Y., Liu, G., Chen, B., Yao, S. (2011). Simultaneous determination of flavanones, hydroxycinnamic acids and alkaloids in citrus fruits by HPLC-DAD-ESI/MS. Food Chemistry, 127, 880-885. [ Links ]
Jiang, M.H., Yang, L., Zhu, L., Piao, J.H. & Jiang, J.G. (2011). Comparative GC/MS analysis of essential Oils extracted by 3 methods from the bud of Citrus aurantium L. var. amara Engl. Journal of Food Science, 76(9), 1219-1225. [ Links ]
Jung, H.K., Jeong, Y.S., Park, C.D., Park, C.H., Hong, J.H. (2011). Inhibitory effect of citrus peel extract on lipid accumulation of 3T3-L1 adipocytes. Journal of the Korean Society for Applied Biological Chemistry, 54(2), 169-176. [ Links ]
Kelebek, H. (2010). Sugars, organic acids, phenolic compositions and antioxidant activity of Grapefruit (Citrus paradisi) cultivars grown in Turkey. Industrial Crops and Products, 32, 269-274. [ Links ]
Kelebek, H & Selli, S. (2014). Identification of phenolic compositions and the antioxidant capacity of mandarin juices and wines. Journal of Food Science and Technology, 51(6), 1094-1101. [ Links ]
Lagha-Benamrouche, S., & Madani, K. (2013). Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria:Peels and leaves. Industrial Crops and Products, 50, 723-730. [ Links ]
Lampila, P., van Lieshout, M., Gremmen, B., & Lähteenmäki, L. (2009). Consumer attitudes towards enhanced flavonoid content in fruit. Food Research International, 42, 122-129. [ Links ]
Ledesma-Escobar, C.A., & Luque de Castro, M.D. (2014). Towards a comprehensive exploitation of citrus. Trends in Food Science and Technology, 39, 63-75. [ Links ]
Levaj, B., Dragovic-Uzelac, V., Bursac-Kovacevic, D., & Krasnici, N. (2008). Determination of flavonoids in pulp and peel of mandarin fruits. Agriculturae Conspectus Scientificus, 74, 3, 221-225. [ Links ]
Li, P.L., Liu, M.H., Hu, J.H., Su, W.W. (2014). Systematic chemical profiling of Citrus grandis 'Tomentosa' by ultra-fast liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 90, 167-179. [ Links ]
Loizzo, M.R., Tundis, R., Bonesi, M., Menichini, F., DeLuca, D., Colica, C., Minichini, F. (2013). Evaluation of Citrus aurantifolia peel and leaves extracts for their chemical composition, antioxidant and anti-cholinesterase activities. Journal of the Science of Food and Agriculture, 92, 2960-2967. [ Links ]
Londoño-Londoño, J., Rodrigues de Lima, V., Lara, O., Gil, A., Crecsynsky Pasa, T.B., Arango, G.J., Ramirez Pineda, J.R. (2010). Clean recovery of antioxidant flavonoids from citrus peel: Optimizing an aqueous ultrasound-assisted extraction method. Food Chemistry, 119, 81-87. [ Links ]
Lorente, J., Vegara, S., Martí, N., Ibarz, A., Coll, L., Hernández, J., Valero, M., & Saura, D. (2014). Chemical guide parameters for Spanish lemon (Citrus limon (L.) Burm.) juices. Food Chemistry, 162, 186-191. [ Links ]
Makovsek, K., Knez, Z., & Skerget, M. (2012). Influence of process parameters on the extraction of flavanones from mandarin peel. Acta Chim Slovenia, 59, 879-887. [ Links ]
Menichini, F., Tundis, R., Loizzo, M.R., Bonesi, M., Liu, B., Jones, P., Persaud, S.J., Mastellone, V., Lombardi, P., Houghton, P.J., Avallone, Menichini, F. (2011a). C. medica cv Diamante peel chemical composition and influence on glucose homeostasis and metabolic parameters. Food Chemistry, 124, 1083-1089. [ Links ]
Menichini, F., Loizzo, M.R., Bonesi, M., Conforti, F., DeLuca, D., Statti, G.A., Cindio, B., Menichini, F., Tundis, R. (2011b). Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. cv Diamante flowers, leaves and fruits at two maturity stages. Food and Chemical Toxicology, 49, 1549-1555. [ Links ]
Mohammad, A., Bhawani, S.A., & Sharma, S. (2010). Analysis of herbal products by thin-layer chromatography: A Review. International Journal of Pharma and Bio Sciences, 1, (2), 1-51. [ Links ]
Montes-Belmont, R. (2009). Diversidad de compuestos químicos producidos por las plantas contra hongos fitopatógenos. Revista Mexicana de Micología, 29, 73-82. [ Links ]
Moulehi, I., Bourgou, S., Ourghemmi, I., & Tounsi, M.S. (2012). Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Industrial Crops and Products, 39, 74-80. [ Links ]
Natarajan, N., Thamaraiselvan, R., Lingaiah, H., Srinivasan, P., Periyasamy, B.M. (2011). Effect of flavonone hesperidin on the apoptosis of human mammary carcinoma cell line MCF-7. Biomedicine & Preventive Nutrition, 1, 207-215. [ Links ]
Neves, A.L., Chinali Komesu, M. & Sala Di Matteo, M.A. (2010). Effects of Green Tea Use on Wound Healing. International Journal of Morphology. Morphol., 2S(3):905-910. [ Links ]
Özçelik, B., Kartal, M., & Orhan, I. (2011). Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharmaceutical Biology, 49(4), 396-402. [ Links ]
Pan, Z., Li, Y., Deng, X., & Xiao, S. (2014). Non-targeted metabolomic analysis of orange (Citrus sinensis [L.] Osbeck) wild type and bud mutant fruits by direct analysis in real-time and HPLC-electrospray mass spectrometry. Metabolomics, 10, 508-523. [ Links ]
Pekal, A., Drozdz, P., & Biesaga, M. (2011). Evaluation of the antioxidant properties of fruit and flavoured black teas. European Journal of Nutrition, 50, 681-688. [ Links ]
Procházková, D., Boušová, I. & Wilhelmová, N. (2011). Antioxidant and prooxidant properties of flavonoids. Fitoterapia, 82, 513-523. [ Links ]
Ramful, D., Tarnus, E., Aruoma, O.I., Bourdon, E., & Bahorun, T. (2011). Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Research International, 44, 2088-2099. [ Links ]
Rodríguez-Rivera, M.P., Lugo-Cervantes, E., Winterhalter, P., & Jerz, G. (2014). Metabolite profiling of polyphenols in peels of Citrus limetta Risso by combination of preparative high-speed countercurrent chromatography and LC-ESI-MS/MS. Food Chemistry, 158, 139-152. [ Links ]
Rousos, P.A. (2011). Phytochemicals and antioxidant capacity of orange (Citrus sinensis (l.) Osbeck cv. Salustiana) juice produced under organic and integrated farming system in. Scentia Horticulturae, 129, 235-258. [ Links ]
Sandoval-Montemayor, N.E., Garcia, A., Elizondo-Treviño, E., Garza-González, E., Alvarez, L., Camacho-Corona, M.R. (2012). Chemical composition of hexane extract of Citrus aurantifolia and Anti-Mycobacterium tuberculosis activity of some of its constituents. Molecules, 17, 11173-11184. [ Links ]
Sanfélix-Gimeno, G., Peiró, S., Librero, J., Ausejo-Segura, M., Suárez-Alemán, C., Molina-López, T., Celaya, M.C., Castaño-Riera, E. (2010). Population analysis by area of health of changes in consumption, price and expenditure of cardiovascular drugs in eight autonomous communities, Spain. Revista Española Salud Pública, 84, 4, 389-407. [ Links ]
Scordino, M., Sabatino, L., Belligno, A., & Gagliano, G. (2011). Flavonoids and furocoumarins distribution of unripe chinotto (Citrus x myrtifolia Rafinesque) fruit: beverage processing homogenate and juice characterization. European Food Research and technology, 233, 759-767. [ Links ]
Soto, M.L., Moure, A., Domínguez, H, & Parajó, J.C. (2011). Recovery, concentration and purification of phenolic compounds by adsorption: A review. Journal of Food Engineering, 105, 1-27. [ Links ]
Soto-Vaca, A., Gutierrez, A., Losso, J.N., Xu, Z., & Finley, J.W. (2012). Evolution of Phenolic Compounds from Color and Flavor Problems to Health Benefits. Journal of Agricultural and Food Chemistry, 60, 6658-6677. [ Links ]
Sun, Y., Qiao, L., Shen, Y., Jiang, P., Chen, J., & Ye, X. (2013). Phytochemical profile and antioxidant activity of physiological drop of citrus fruits. Journal of Food Science, 78(1), C37-C42. [ Links ]
Tham, W.W. & Liew, K.C. (2014). Influence of different extraction temperatures and methanol solvent percentages on the total phenols and total flavonoids from the heartwood and bark of Acacia auriculiformis. European Journal of Wood and Wood Products, 72, 67-72. [ Links ]
Vikram, A., Jayaprakasha, G.K., Jesudhasan, P.R., Pillai, S.D. & Patil, B.S. (2010). Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. Journal of Applied Microbiology, 109, 515-527. [ Links ]
Wang, S., Chen, P., Jiang, W., Wu, L., Chen, L., Fan, X., Wang, Y. & Cheng, Y. (2014). Identification of the effective constituents for anti-inflammatory activity of Ju-Zhi-Jiang-Tang, an ancient traditional Chinese medicine formula. Journal of Chromatography A, 1348, 105-124. [ Links ]
Xi, W., Fang, B., Zhao, Q., Jiao, B., & Zhou, Z. (2014a). Flavonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chemistry, 161, 230-238. [ Links ]
Xi, W., Zhang, Y., Sun, Y., Shen, Y., & Zhou, Z. (2014b). Phenolic composition of Chinese wild mandarin (Citrus reticulate Blanco.) pulps and their antioxidant properties. Industrial Crops and Products, 52, 466-474. [ Links ]
Ye, X.Q., Chen, J.C., Liu, D.H., Jiang, P., Shi, J., Xue, S., Wu, D., Xu, J.G., & Kakuda, Y. (2011). Identification of bioactive composition and antioxidant activity in young mandarin fruits. Food Chemistry, 124, 1561-1566. [ Links ]
Yoo, K., Hwang, I.K., Park, J.H., & Moon, B. (2009). Comparative flavonoids contents of selected herbs and associations of their radical scavenging activity with antiproliferative actions in V79-4 Cells. Journal of Food Science, 74, 6, C462-468. [ Links ]
Yu, E.A., Kim, G.P., Jeong, S.W., Park, S., Lee, S.J., Kim, J.H., Lee, W.S., Bark, K.M., Jin, J.S., & Shin, S.C. (2014). Flavonoid profile and biological activity of Korean citrus varieties (II): Pyunkyul (Citrus tangerina Hort. ex Tanaka) and overall contribution of its flavonoids to antioxidant effect. Journal of Functional Foods, 6, 637-642. [ Links ]
Yu, M.W., Lou, S.N., Chiu, E.M., & Ho, C.T. (2013). Antioxidant activity and effective compounds of immature calamondin peel. Food Chemistry, 136, 1130-1135. [ Links ]
Zang, L., Shimada, Y., Kawajiri, J., Tanaka, T., & Nishimura, N. (2014). Effects of Yuzu (Citrus junos Siebold ex Tanaka) peel on the diet-induced obesity in a zebrafish model. Journal of Functional Food, 10, 499-510. [ Links ]
Zhang, M., Duan, C., Zang, Y., Huang, Z., & Liu, G. (2011). The flavonoid composition of flavedo and juice from the pummelo cultivar (Citrus grandis (L.) Osbeck) and the grapefruit cultivar (Citrus paradisi) from China. Food Chemistry, 129, 1530-1536. [ Links ]
Zhang, M., Jiang, S., Qian, D., Shang, E.X., & Duan, J.A. (2014a). Determination of metabolism of neohesperidin by human intestinal bacteria by UPLC-Q-TOF/MS. Chromatographia, 77, 439-445. [ Links ]
Zhang, M., Nan, H., Wang, Y., Jiang, X., & Li, Z. (2014b). Comparison of Flavonoid compounds in the flavedo and juice of two pummelo cultivars (Citrus grandis L. Osbeck) from different cultivation regions in China. Molecules, 19, 17314-17328. [ Links ]
Zhang, Y., Sun, Y., Xi, W., Shen, Y., Qiao, L., Zhong, L., Ye, X., & Zhou, Z. (2014c). Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chemistry, 145, 674-680. [ Links ]