INTRODUCTION
Pigment production using microorganisms can be easily controlled and performed with inexpensive raw materials and minor environmental costs, as some agroindustrial waste products can be used as substrates (Panesar, Kaur, & Panesar, 2015). Bacteria, fungi, and algae have been used to produce pigments such as carotenoids, melanins, flavins, quinones, and prodigiosins, and many of these have shown industrially and pharmaceutically relevant biological activities (Venil, Zakaria, & Ahmad, 2013).
Some bacterial pigments have been obtained by different species of the genera Bacillus, Pseudomonas, Streptomyces and Serratia (Babitha, 2009). Belonging to the family Enterobacteriaceae, Serratia marcescens is a facultative gram-negative rod-shaped bacterium that can be isolated from water, soil, plants and insects, and can grow in culture media containing different carbon sources (Casullo de Araújo, Fukushima, & Campos Takaki, 2010; Giri, Anandkumar, Muthukumaran, & Pennathur, 2004). Some strains of S. marcescens produce a red pigment called prodigiosin (5-[(3-methoxy-5-pyrrol-2-ylidene-pyrrol-2-ylidene)-methyl]-2-methyl-3-pentyl-1H-pyrrole), which is a secondary metabolite possessing a unique chemical structure that confers it with a wide range of biological activities (Giri et al., 2004; Venil & Lakshmanaperumalsamy, 2009).
Prodigiosin reportedly has biocidal activity against Radopholus similis and Meloidogyne javanica, two plant parasitic nematodes (Rahul et al., 2014). It also inhibits cell proliferation by inducing apoptosis in different human cancer cell lines with no toxic effects on normal cells (Dalili et al., 2012; Kamble & Hiwarale, 2012; Kavitha, Aiswariya, & Ratnavali, 2010). In addition, it can alter mitochondrial functions in Trypanosoma cruzi, possibly indicating antiparasitic activity (Baquero, Echeverri, Maya, & Triana, 2011).
Furthermore, prodigiosin reportedly inhibits the growth of plant pathogenic fungi such as Pythium ultimum and Fusarium oxysporum, bacteria such as Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella typhi and Escherichia coli, and some algae (Alihosseini, Ju, Lango , Hammock, & Sun, 2008; Kamble & Hiwarale, 2012; Roberts et al., 2007; Suryawanshi, Patil, Borase, Salunke, & Patil, 2014).
Prodigiosin production by S. marcescens occurs both under aerobic and anaerobic conditions and depends on various factors, such as bacterial strain, temperature and time of incubation, aeration, and agitation of cultures, and sources of nitrogen and carbon, concentration of inorganic salts, and pH of culture medium (Suu Tsou, & Liu, 2011; Suryawanshi et al., 2014). Several culture media have been tested for metabolites and enzymes production by Serratia spp. Reportedly, culture media with high protein and complex carbohydrates concentrations induce prodigiosin production; whereas the presence of simple carbohydrates promotes cell growth but decreases pigment production (Suryawanshi et al., 2014).
Prodigiosin has been previously obtained using stirring rates of 100-200 rpm, pH of 6.0-9.0, and temperatures starting at 20°C; however, its production is reportedly completely inhibited above 37°C. It has also been produced using synthetic and complex culture media containing different carbon and nitrogen sources, such as glucose, casein, peptone, sucrose, glycerol, tryptone, and yeast extract. Agroindustrial wastes and other substrates, such as seed extracts, oils, maize flour, gelatin, wastewater from cassava-processing industries, and liquors obtained from maize maceration have also been used (Aruldass, Venil, Zakaria, & Ahmad, 2014; Casullo de Araújo et al., 2010; Giri et al., 2004; Kim, Kim, & Hong, 1999; Song et al., 2006; Su et al., 2011; Toro Álvarez, Patiño Naranjo, & Ríos Estepa, 2001; Venil & Lakshmanaperumalsamy, 2009; Wei & Chen, 2005).
In this study, prodigiosin production by S. marcescens using culture media with different pH values and concentrations of protein substrates rich in casein obtained through acid-induced precipitation of milk was described. Prodigiosin production in a stirred-tank bioreactor was assessed and the effect of supplementation of culture media with simple sugars on prodigiosin yield and its antimicrobial activity was assessed.
MATERIALS AND METHODS
Microorganism
A strain of S. marcescens which was obtained from a human infected tissue showing pigmentation in Luria-Bertani (LB) agar (Bertani, 1951) was used. The strain was donated by the Faculty of Health Sciences of Institución Universitaria Colegio Mayor de Antioquia, Medellin, Colombia. It was identified by the API20E system (bioMérieux®, Marcy-l'Étoile, France) according to the manufacturer’s instructions and by sequencing the 16S rRNA gene. The strain was kept in brain heart infusion (BHI) broth culture with glycerol at (50°C and reactivated before each test by incubation in LB agar at 37°C for 24 hours.
Molecular characterization
DNA was extracted from a BHI broth culture after 12 hours of incubation at 37°C using the Wizard® genomic DNA purification kit (Promega Co., Madison, WI, USA) according to the manufacturer’s instructions. Polymerase chain reaction (PCR) amplification of the 16S rRNA gene was performed using the universal primers for bacteria 27f (5'-AGAGTTTGATCMTGGCTCAG-3') and 1492r (5'-TACGGYTACCTTGTTACGACTT-3') (Lane, 1991). The final volume for each reaction was 50 μL; the mixture contained 10 μL of 5X buffer, 1.25 U of Taq polymerase (GoTaq® Flexi; Promega), MgCl2 (2 mmol/L), deoxynucleotide triphosphates (0.2 mmol/L), each primer with a concentration of 8 μmol/L, and template DNA quantified in Qubit® 3.0 fluorometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at ~ 4 ng/μL. The following PCR conditions were used: initial denaturation at 94°C for 1 min followed by 30 cycles consisting of denaturation at 94°C for 1 min, alignment of primers at 52°C for 1 min and extension at 72°C for 1 min, and a final extension for 10 min. The presence of PCR products was confirmed by agarose gel electrophoresis. Sequencing was performed by Macrogen Inc. and the result was compared to 16S rRNA gene sequences from the GenBank database using BLAST (http://www.ncbi.nlm.nih.gov/).
Culture conditions
The culture media contained MgSO4(7H2O (1 g/L), NaCl (1 g/L), and K2HPO4 (1 g/L) (Kim, Kim, & Hong, 1999). The carbon and nitrogen source was casein, either high-purity (Sigma-Aldrich, Co.) or that obtained through vinegar-induced precipitation of bovine milk. To obtain the latter, a protocol was standardized in this work; commercial white vinegar (4% acidity, pH 2.3 and potassium metabisulfite as a preservative) was added to pasteurized liquid skim milk, and the precipitate was filtered, washed once with distilled water, and dried at 60°C for 24 hours. The dried casein obtained was ground to an average particle size of 0.590 mm. The media were autoclaved at 120°C for 15 min. Cultures were performed in 250 mL Erlenmeyer flasks with 50 mL medium, with shaking at 120 rpm and incubation at 22 (2°C for 24 hours. The initial pH values were adjusted to 7.0, 8.0, and 9.0 with NaOH or HCl (1 mol/L) and casein concentrations of 2.5, 5, and 10 g/L were evaluated following a completely randomized design with factorial arrangement with three replicates. To prepare the inoculum, S. marcescens was cultured on LB agar for 24 hours at 37°C and some colonies were resuspended in peptone water until an optical density corresponding to a 0.5 McFarland standard (1.5(108 cells/mL) was attained. Bacterial suspension was added to each flask, and the inoculum size was 1% of the total volume.
Pigment extraction and pigment and cell growth quantification
To extract the pigment, a sample of 10 ml of culture for each evaluated condition were centrifuged at 2500 g for 20 min and the supernatant was discarded; the pellet was resuspended in 10 mL acidified methanol with 4% (v/v) of 1 mol/L HCl and centrifuged again; the supernatant was stored and its absorbance was determined at 500 nm (Nanocolor ®, Macherey-Nagel Inc.) (Kamble & Hiwarale, 2012). Cell growth was quantified by optical density of the culture at 600 nm.
Effect of glucose concentration on pigment production and antimicrobial activity
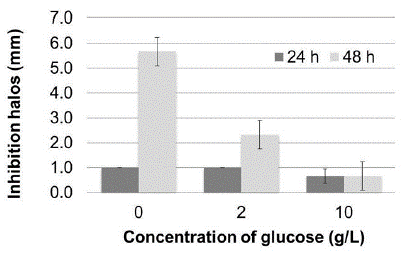
Tests were performed under the abovementioned culture conditions, with culture media containing 10 g/L casein precipitated with vinegar and supplemented with 0, 2, or 10 g/L glucose at pH 8.0. A completely random design with three repetitions was followed. Samples were taken after 24 and 48 hours of incubation and the pigment was extracted and quantified.
To determine the antibacterial activity, extracts were concentrated by vacuum evaporation at 45°C until all solvent was removed. Next, the pigment was resuspended in 500 μL DMSO. The S. aureus strain ATCC 29213 was used as the test microorganism to assess antimicrobial activity, and it was reactivated at 37°C on LB agar for 24 hours before the antagonism test. A cell suspension with optical density adjusted to a 0.5 McFarland standard was prepared from this culture. The activity was determined by disk diffusion on Müeller-Hinton agar; three 0.5 mm diameter filter paper (Whatman® No. 1) impregnated with 10 μL of one of the obtained pigments, were placed on a plate inoculated with the test microorganism. The diameters of inhibition halos were measured after the plates were incubated at 37°C for 24 hours (Gulani, Bhattacharya & Dast, 2012). A gentamicin solution (1 mg/mL) was used as positive control.
Pigment production in stirred-tank bioreactor
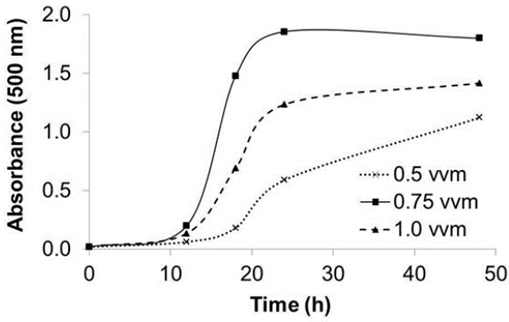
Cultures were performed in a 10 L stirred-tank with a diameter of 30 cm with a Rushton turbine and a perforated ring sparger coupled to a flow meter. Aeration was provided by feeding sterilized compressed air (15 psi). The culture medium was prepared with 10 g/L casein precipitated with vinegar from milk and adjusted to a pH value of 8.0. The effective volume of culture medium was 4 L, the stirring speed was 400 rpm, and the temperature was maintained at 25 ± 2°C for 48 hours. The inoculum was prepared as indicated for cultures in Erlenmeyer flasks; the inoculum size was 1% (v/v). Aeration rates of 0.5, 0.75, and 1 vvm were evaluated.
Statistical analysis
To evaluate the effect of casein type, substrate concentration, and pH on prodigiosin production, a multivariate variance analysis and a comparison of means by Tukey's HSD multiple comparisons test were performed (95% confidence level) using Statgraphics® Centurion XVI. The effect of glucose concentration on pigment production was evaluated similarly. Before any statistical analysis, the homogeneity of variance and the normality of the obtained data were verified.
RESULTS AND DISCUSSION
Microorganism identification
A gram-negative, oxidase-negative bacillus forming red colonies characteristic of Serratia species, such as S. plymuthica, S. rubidea and S. marcescens (Kalbe, Marten, & Berg, 1996) was used. The isolate was identified as S. marcescens with a 94% of identification accuracy using the API20E system (bioMérieux®), and its identity was confirmed by analyzing 16S rRNA gene sequence similarity. The fragment length, analyzed by BLAST alignment, was 1230 bases and coincided 98% with the partial sequence of the 16S rRNA gene of the S. marcescens isolate RTL-100 (accession number HE995790).
Effect of casein type and initial pH on cell growth and pigment production
Biomass increased in all culture media prepared with both casein types, especially when using higher substrate concentrations. However, the highest cell growth occurred when casein precipitated from milk with vinegar was used as substrate and initial pH of cultures did not affect the bacterial growth (figure 1). No significant absorbances at 500 nm were detected for the supernatants of the centrifuged cultures. These results are consistent with those of previous studies, which reported that prodigiosin does not diffuse in the medium and is often located within vesicles or is adhered to the inner membrane of bacteria (Venil & Lakshmanaperumalsamy, 2009). Figure 2 shows pigment production in Erlenmeyer flasks using different casein concentrations, either highly pure or precipitated with vinegar, at different pH values. Increased pigment production was achieved at higher casein concentrations and when casein precipitated with vinegar was used as substrate.
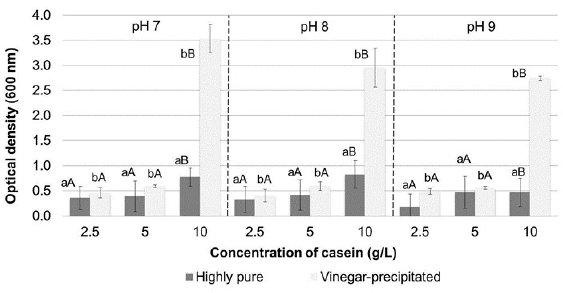
Figure 1 Cell growth in Erlenmeyer flasks expressed as optical density at 600 nm of the cultures. The bars represent the mean value (n = 3 replicates). Different lowercase letters indicate significant differences between the casein type (commercial or vinegar-precipitated), and different uppercase letters denote significant differences between the substrate concentration, with 95% confidence.
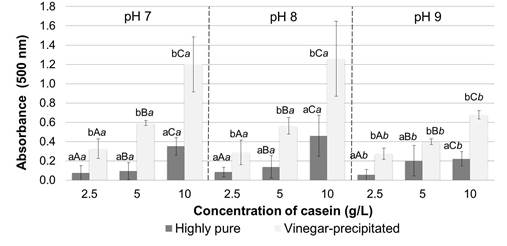
Figura 2 Prodigiosin production in Erlenmeyer flasks expressed as absorbance at 500 nm of the extracts obtained. The bars represent the mean value ( n= replicates). Different lowercase letters indicate significant differences between the casein type ( commercial or vinegar-precipitated), different uppercase letters denote significant differences between the substrate concentration and different cursive letters. indicate differences between the initial pH, all with 95% confidence.
The multivariate analysis of variance performed (table 1) indicated that there were significant differences in prodigiosin production for each variable evaluated. Comparison of means confirmed that there was higher pigment production when casein precipitated with vinegar was used as a substrate and when the substrate concentration was 10 g/L. There were no significant differences between prodigiosin production with initial pH values of 7.0 and 8.0; however, a significantly higher pigment yield was obtained compared to the pH 9.0 treatment (figure 2).
Table 1 Analysis of variance for prodigiosin production to determine the effect of casein type, casein concentration, and pH
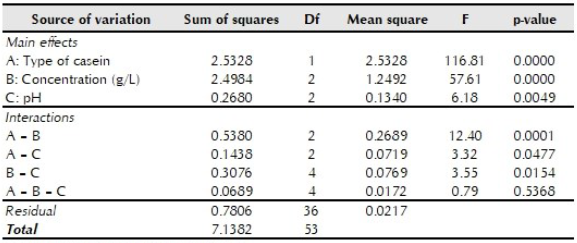
p < 0.05 indicates statistical significance.
S. marcescens can grow in culture media with initial pH values ranging from 5.0 to 9.0 (Imhoff, 2005). It has already been reported that prodigiosin production is associated with the increase in biomass (Haddix & Shanks, 2018) and this was confirmed in the present study when an increase in biomass and pigment production was observed for the three initial pH values assessed, although to a lesser extent in media with an initial pH value of 9.0.
Unlike several gram-negative bacteria, S. marcescens can use casein as a carbon source due to the action of its metalloproteinases on the extracellular matrix (Matsumoto, Maeda, Takata, Kamata, & Okamura, 1984; Pakhale & Bhagwat, 2016), which explains the growth obtained in the culture media studied. In addition, S. marcescens can obtain tryptophan and other amino acids such as proline, serine, and glycine, from the proteins it uses as a substrate; these amino acids have been associated with pigment production in various microorganisms and act as precursors during prodigiosin production (Aruldass et al., 2014; Bharmal, Jahagirdar, & Aruna, 2012).
S. marcescens reportedly uses casein as a nitrogen source more effectively than meat extract, sodium nitrate, ammonium sulfate or urea (Suryawanshi et al., 2014). In this study, casein was the limiting substrate in pigment production, as biomass and prodigiosin production increased with higher concentrations of casein in the culture media. Other studies have reported that casein concentration can be as high as 20 g/L without inhibiting the microorganism (Song et al., 2006).
There was a higher pigment production in media prepared with casein precipitated with vinegar than in media prepared with high purity casein. Although the pH of milk is lowered until the isoelectric point of casein is reached, other proteins and peptides with similar isoelectric points can co-precipitate (Yen, Lin, et al., 1996) & Tu, 2015) and act as additional nutrients present in the medium.
High-purity casein production through acid-induced precipitation requires washing the precipitate several times. Because the precipitate was washed only once in this study, other components, such as some fatty acids, ions, and peptides, could be associated with the precipitated protein and also increase the growth of S. marcescens (Jensen, 1995; Southward, nd). Supplements with oleic acid, triolein, and even sources of mixed vegetable oils reportedly improve prodigiosin production. In addition, the presence of acetic acid in the precipitated casein could also potentiate pigment production, as bacteria condense acetate to form the pyrrole ring and the side chain of prodigiosin (Bharmal et al., 2012; Venil & Lakshmanaperumalsamy, 2009).
Initial pH values also affected prodigiosin production. Prodigiosin is reportedly synthesized by S. marcescens more efficiently at pH values above 6.0 (Venil & Lakshmanaperumalsamy, 2009). In this study, a significantly lower pigment yield was obtained when initial pH value of 9.0 was used, due to the low cell growth at this pH value. This difference in pigment production was more noticeable for media prepared with casein precipitated with vinegar and for high substrate concentrations.
Effect of glucose supplement
Prodigiosin production was evaluated using casein precipitated with vinegar as substrate, with and without glucose supplementation. As shown in figure 3, a greater amount of pigment was produced after 48 hours when no glucose was added to the culture medium. Table 2 shows a significant effect of glucose concentration and incubation time on pigment production, in addition to the interaction between these variables.
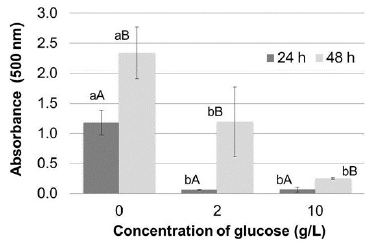
Figure 3 Effect of glucose supplement on prodigiosin production in Erlenmeyer flasks expressed as absorbance at 500 nm of the extracts obtained. The bars represent the mean value (n = 3 replicates). Different lowercase letters indicate significant differences between concentration of glucose and different uppercase letters denote significant differences between the incubation time, all with 95% confidence.
Table 2 Analysis of variance for prodigiosin production to determine the effect of supplementation with glucose and incubation time
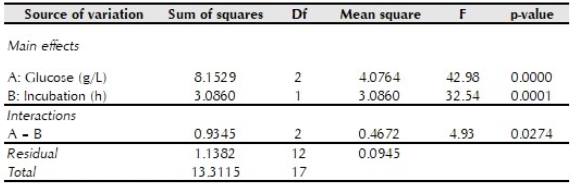
p < 0.05 indicates statistical significance
Comparison of means confirmed that supplementation of glucose to the culture medium significantly decreased pigment yield, higher prodigiosin yields were obtained after an incubation for 48 hours; and pigment production was not significantly different between culture media with 2 or 10 g/L glucose incubated for 24 hours.
Although glucose can be assimilated by S. marcescens more rapidly than other substrates, it reportedly inhibits prodigiosin production by increasing glucose-6-phosphate dehydrogenase levels (Gargallo, Lorén, Guinea, & Viñas, 1987). Prodigiosin production reportedly decreases by 60% when glucose is used as the only carbon source compared to when more complex substrates, such as starch or casein, are used (Suryawanshi et al., 2014). High intracellular glucose contents can suppress the synthesis of second messengers, such as cyclic AMP which functions as a catabolic regulator controlling the expression of certain genes, some of them associated with prodigiosin production (Fender et al., 2012). In this study, pigment production decreased by at least 50% when 2 g/L glucose was added to the culture media.
Antimicrobial activity against S. aureus was also affected by the glucose concentration (figure 4). Antimicrobial activity increased when no glucose supplement was added to the culture medium and after 48 hours of incubation, indicating that activity and pigment production are directly related. An increased antibacterial activity of prodigiosin against gram-positive microorganisms, especially against S. aureus and B. subtilis, has been demonstrated previously and could be verified in this study. Growth inhibition of some gram-negative bacteria would require very high pigment concentrations, whereas other bacteria are not affected by prodigiosin (Bhuryal et al., 2012; Suryawanshi et al., 2014).
Pigment production in a stirred-tank bioreactor
Three aeration rates were used to evaluate the effect of airflow on prodigiosin production in a stirred tank bioreactor. Figure 5 shows that the highest pigment yield was achieved at 0.75 vvm aeration. Consistent with other studies, this finding indicates that the prodigiosin yield is higher if high oxygen transfers are guaranteed. However, pigment production may be lower when very high aeration rates are used (Heinemann, Howard, & Palocz, 1970; Sumathi, Mohanapriya, Swarnalatha, Dinesh, & Sekaran, 2014).
CONCLUSIONS
Prodigiosin production with S. marcescens using casein as substrate is affected by the initial pH and casein concentration. In this study, highest pigment production was obtained at pH 8.0 when the substrate in culture medium was 10 g/L casein obtained through acid-induced precipitation of milk. Pigment yield was higher with vinegar-precipitated casein than high-purity casein. There is a direct relationship between S. aureus inhibition and prodigiosin concentration; both decreased when culture media was supplemented with glucose. Likewise, the aeration rate affected the prodigiosin production in a stirred-tank bioreactor. The results obtained in our study provide information about the use of novel substrates in microbial pigment production.