INTRODUCTION
Striped catfish (Pseudoplatystoma magdaleniatum) is the most commercially valuable fish species in the Magdalena river basin and is subjected to excessive fishing pressure. During year 1977, it represented 37% of the total catch in this basin. Nine years later, it only accounted for 9% (Mójica et al., 2012). Catching volumes have decreased by more than 90% in the last 40 years (Mójica et al., 2012) and 60% of the captured individuals are under the minimum legal size (Valderrama et al., 2014). The striped catfish is reported in the Red Book of Freshwater Fishes of Colombia as a critically endangered species due to the strong fishing pressure on this resource and the environmental deterioration of its habitat (Mojica et al., 2012).
An important problem for striped catfish production in captivity is the asynchrony between males and females during the reproductive season: when males are in the spermiation phase and females are still immature. Semen cryopreservation can be utilized for the reproduction of species with asynchronous gonadal maturation and seasonal reproductive cycles (Lahnsteiner et al., 2004), allowing the preservation of threatened or endangered species (Bobe and Labbé, 2009; Horváth et al., 2012). Furthermore, semen cryopreservation is a safe method to store and preserve genetic material (Cabrita et al., 2014) for fingerling production in the fish industry. It helps to meet the demand for semen and simplifies reproduction in captivity, particularly when there are mature eggs but no semen available (Tiersch, 2008). It can also help to reduce the pressure exerted by fishing (Medina et al., 2005).
The procedure of semen collection, composition of the cryoprotective solution, percentage of cryoprotectant inclusion, and freezing and thawing curves are important variables for the success of semen cryopreservation protocols (Irawan et al., 2010; Tiersch, 2011). Diluents and cryoprotectants (internal and external) are used to increase the ejaculate volume and protect the sperm from toxic cell metabolites and sudden changes in temperature (Medina et al., 2005). Cryoprotectants must be safe and highly soluble in water (Denniston et al., 2011). Cryoprotectants maintain cellular viability, preventing damage during freezing and thawing (Ramírez et al., 2011). Dimethyl sulfoxide (DMSO), methanol, propylene glycol, dimethylacetamide (DMA), and ethylene glycol (ETG) are commonly used cryoprotectants (Irawan et al., 2010; Ramírez et al., 2011; Routray et al., 2007). Antioxidants are also added to the cryoprotective solution to reduce the harmful effects of reactive oxygen species (ROS) released during cryopreservation (Navarro et al., 2012; Butts et al., 2011). Vitamin E (α-tocopherol and its derivatives) is used as an antioxidant in cryopreservation of fish semen to control the effects of ROS, thus protecting against oxidative stress (Navarro et al., 2014).
The literature is scarce regarding semen cryopreservation and cryoprotectants suitable for Pseudoplatystoma magdaleniatum. Atencio et al. (2016), recommended to include, between 6 and 8% DMSO. Other studies using P. fasciatum semen suggested DMSO inclusions equal to or greater than 10% drastically reduce sperm viability (Pinzón et al., 2005; Guarnizo, 2007). These last studies were conducted before the genus was revised by Buitrago-Suarez and Burr (2007) who established that P. fasciatum is not reported for Colombia; thus, the species evaluated by Pinzón et al. (2005), and Guarnizo (2007) was probably P. metaense considering they used specimens of striped catfish from the Meta river basin.
Reliable semen cryopreservation protocols are required to improve Pseudoplatystoma magdaleniatum reproduction. This would increase the availability of fingerlings for restocking programs and the promotion of its cultivation. The objective of the present study was to evaluate semen cryopreservation of striped catfish using two inclusion levels (5 and 10%) of DMSO, DMA, and ETG.
MATHERIALS AND METHODOS
BIOLOGICAL MATERIAL
The study was conducted at San Silvestre SA Fish Station (PSS) located in Barrancabermeja, Santander (Colombia). The station is located at 7°04'03" latitude north, 73°50'50" longitude west. Altitude is 75 masl, with 28.4 °C average annual temperature. Striped catfish (P. magdaleniatum) males captured in the middle Magdalena River (Barrancabermeja) were used, adapted and maintained under captive conditions in earth ponds at PSS, at a density of 0.5 kg/m2, at least during one year.
Five males were selected during the spermiation phase, which is characterized by the release of seminal fluid when a slight abdominal pressure is applied. The selected specimens were transferred to rectangular tanks (6m3 total volume) for adaptation to the experimental conditions, reduce the stress caused by manipulation, and change of environment for the application of hormonal inducers intended to increase the seminal volume. The induction was performed with salmon analogue GnRH + domperidone (Ovaprim®, Syndel, Canada) using a single dose of 0.4 ml/kg live weight (Atencio, 2001).
All procedures involving animal handling were performed in accordance with the standards for the use of laboratory animals described by the Committee on Care and Use of Laboratory Animal Resources of the National Research Council (National Academies, USA).
The semen was collected in 50 ml sterile and dry Falcon tubes between 12 and 14 hours' post-induction at 28±0.5°C water temperature after abdominal massage in cranial-caudal direction. Prior to the collection, gentle pressure was applied on the urogenital papilla, with the aim of eliminating remains of water, urine or feces to avoid contamination of the semen.
EVALUATION OF FRESH, PRE-FROZEN AND CRYOPRESERVED SEMEN
Semen was immediately assessed after collection to establish fresh semen quality, used as the reference (control) to evaluate the collection process and compare with the pre-frozen (semen diluted in the cryoprotective solution before freezing) and thawed semen.
Volume and color. Semen volume was measured directly in 50 ml Falcon tubes. Color was recorded using the RossPope color scale, and samples contaminated with blood, feces or urine were discarded (Atencio et al., 2014).
Total motility, motility type, total progressivity and sperm velocity. A semen sample (0.25 μl) was placed in a Makler counting chamber (Sefi Medical Instruments Ltd, Israel) and activated with 75 μl distilled water (1: 300 dilution), homogenized, and motility (total and types) was measured between 10 and 20 seconds post-activation and expressed as percentage of fast, medium, slow and static sperm using a phase-contrast optical microscope (Nikon, E50i, Japan) and the computer-assisted Sperm Class Analyzer (SCA®) program (Microptic SL, SCA® VET 01, Ver 4.0, Spain). Rapid sperm were those with velocities greater than 100 μιτι/s, medium those with speeds between 45 and 100 μm/s, slow with speeds between 10 and 45 μm/s, and static those sperm without movement. Curvilinear (VCL) and linear (VSL) velocities and total progressivity were also estimated with the same program (SCA®) and expressed in μm/sec.
Activation time. It was evaluated only in fresh semen, in a Makler chamber in which a 0.25 μl fresh semen sample was placed and activated with 75 μl-distilled water (dilution 1: 300). The samples were observed in a phase-contrast optical microscope (Nikon, Eclipse 50i, Japan) with 100x magnification. Activation time was measured from the moment the activating solution (distilled water) was added to the semen sample until approximately 90% of the sperm stopped moving.
Sperm concentration. Semen (1 μl) was mixed with 699 μl of 6% glucose (1: 700 dilution) in a 2 ml Eppendorf and homogenized for five seconds in a vortex at 1200 rpm (Velp Scientifica, Zxclasic, China). Then, 10 μl was placed in a Makler chamber to determine concentration with the SCA®. All samples of fresh semen from each male (n = 5) were analyzed in triplicates.
TREATMENTS AND PREPARATION OF DILUENTS
The semen was cryopreserved with 5 or 10% DMSO, DMA or ETG as internal cryoprotectant (Sigma Chemical Co., St. Louis, MO, USA), and combined with 3% skim milk powder (Merck) as external cryoprotectant and 6% glucose (Sigma Chemical Co., St Louis, MO, USA) (w/v). In total, six cryoprotective solutions were evaluated and fresh semen was used as a control to compare the quality of the pre-frozen and thawed semen.
To prepare the cryoprotective solution, the glucose was mixed with pre-heated (60 °C) distilled water. Then, the corresponding volume (5 or 10% v/v) of internal cryoprotectant was added. Then, the 3% (w/v) skim milk powder and 0.4% (w/v) vitamin E was added. Finally, the volume was completed with distilled water. For DMSO, skim milk powder was added only after the exothermic reaction occurred.
Packing, freezing and thawing. Semen and diluent were mixed at room temperature (28 ± 1 °C) in 1:3 ratios and packed in 2.5 ml straws (Minitüb, Abfül - und Labortechnik GmbH & Co) sealed with polyvinyl and stainless steel spheres. Six semen straws were obtained per treatment and each one was evaluated in triplicate. After reaching equilibrium (10 minutes) the straws were frozen in a dry thermos with nitrogen vapors (MVE 4/2V, AL, USA) for 30 minutes and then transferred to a storage thermos (MVE 24/12V, AL, USA), submerging them directly in liquid nitrogen (Cruz-Casallas et al., 2006; Viveiros et al., 2015). The cryopreserved semen straws were thawed by direct immersion in a serological water bath (Memmert, WNB 7-45, Germany) at 35 °C for 90 seconds (Atencio-García et al., 2017).
STATISTICAL ANALYSIS
We used a 3x2 factorial design (three cryoprotectants and two inclusion percentages). All the variables were subjected to normality tests (Shapiro Wilk test) and variance homogeneity (Bartlett test). Cryoprotectant effect factor (A), percentage of inclusion factor (B), and its interaction (AxB) were analyzed for those variables that fulfilled the mentioned assumptions. A Tukey`s multiple range test was performed to compare the treatments. The criterion for significant difference (*) was p <0.01, while p <0.05 denoted very significant difference (**). Data were expressed as average values ± standard deviation (SD). The statistical analysis was performed with the R Project software, version 3.4.1.
RESULTS
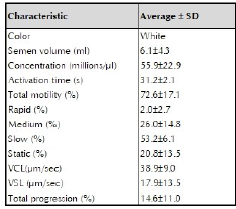
The evaluation of fresh semen showed the following results: 6.1 ml average seminal volume, with 72.6 ± 17.1% average motility, low percentage of sperm with rapid motility (2.0 ± 2, 7%), and high percentage of slow sperm (53.2 ± 6.1%). Average sperm activation time was 31.2 ± 2.1 seconds (table 1).
QUALITY OF PRE-FROZEN SEMEN
Table 2 shows the quality of the semen diluted in the cryoprotective solutions (DMSO, DMA, ETG) at two inclusion levels (5 or 10%) before freezing.
Table 2 Pre-frozen semen quality of striped catfish (Pseudoplatystoma magdaleniatum).
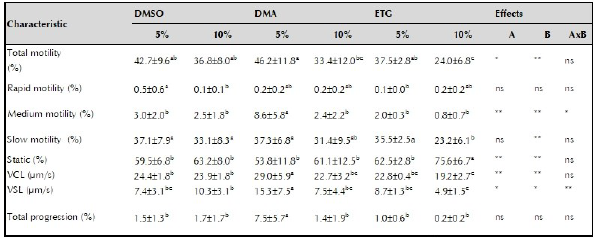
DMSO: dimettaIsulfoxide: DMA: dimethylacetamide: ETC: ethylene glycol: VCL: curvilinear velocity: VSL: linear velocity: A: cryoprotectant effect B: inclusion level effect AxB: factor by factor interaction: * significant difference (p <0.05); ** very significant difference (p <0.01);. ns: not significant difference. Different superscript letters in the same row indicate significant difference (p <0.05).
The type of cryoprotectant (p <0.05) and its level of inclusion affected total motility (p <0.01), and no interaction was observed between them. The DMA 5% treatment resulted in the highest total motility (46.2 ± 11.8%) and ETG 10% in the lowest (24.0 ± 6.8%) among treatments (p < 0.05).
The main factors or their interaction did not affect fast motility sperm (%) or total progressivity. whereas, medium motility sperm (%) was affected by the cryoprotectant (p <0.01), its level of inclusion (p <0.01), and the interaction between both (p <0.05); with DMA 5% resulting in the highest percentage (8.6 ± 5.8%).
Inclusion level affected slow sperm motility (p <0.01) while cryoprotectant type or their interaction did not affect it; with ETG 10% resulting in the lowest percentage of slow sperm motility (23.2 ± 6.1%). The percentage of static spermatozoa was affected by the cryoprotectant (p <0.01) and its inclusion level (p <0.01), but not by their interaction. Semen treated with ETG 10% resulted in the highest percentage of static sperm (75.6 ± 6.7%). The VSL was affected both by the main factors (p <0.05) and their interaction (p <0.01); while the VCL was affected by the factors (p <0.01) but not by their interaction.
QUALITY OF POST-THAWED SEMEN
Table 3 presents the quality after thawing of semen cryopreserved with DMSO, DMA or ETG at two inclusion levels (5 or 10%). Total motility was not affected by the type of cryoprotectant, nor by its inclusion level, but by their interaction (p <0.05). The lowest total motility was observed when semen was treated with 5% ETG (10.3 ± 6.8%) (p <0.05). The VSL and the percentage of rapid, slow, and progressivity sperm were not affected by the main factors or by their interaction.
Table 3 Post-thawed semen quality of striped catfish (Pseudoplatystoma magdaleniatum).
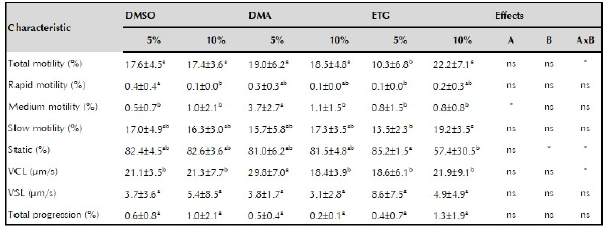
DMSO: dimethylsulfoxide: DMA: dimethylacetamide: ETC: ethylene glycol: VCL: curvilinear velocity; VSL: linear velocity: A: cryoprotectant effect B: inclusion level effect AxB: factor by factor interaction: * significant difference (p <0.05); ** very significant difference (p <0.011: ns: not significant difference. Different superscript letters in the same row indicate significant difference (p <0.05).
The percentage of sperm with medium motility was affected by the type of cryoprotectant, but not by the percentage of inclusion or their interaction. The highest percentage of medium motility sperm was obtained with 5% DMA (3.7 ± 2.7%) (p <0.05). The percentage of static sperm was not affected by the type of cryoprotectant, but it was by the percentage of inclusion and the interaction. The semen treated with 10% ETG resulted in the lowest percentages (57.4 ± 30.5%) of static sperm (p> 0.05).
DISCUSSION
Semen suffered damage when it came in contact with the cryoprotective solutions, causing reduction of total motility, decrease in medium velocity sperm (50-100 μιη/s), increase in static sperm and decrease in VCL and VSL. These variables were affected by the type of cryoprotectant and the level of inclusion; the higher the percentage of inclusion, the greater the deterioration of seminal quality. The pre-frozen semen shows a reduction in total motility between 36.4% (5% DMA) and 66.9% (10% ETG) in relation to the average motility of fresh semen (72.6 ± 17.1%). Only percentage of rapid-moving spermatozoa (greater than 100 μm/s) and total progressivity were not affected either by the factors evaluated (type of cryoprotectant and percentage of inclusion) or by their interaction.
The total motility and its types (rapid, medium, slow and static) of the cryopreserved semen is a sperm quality criterion that allows to measure the success or failure of the cryopreservation process. Padilla (2014) pointed out that from the moment the semen comes into contact with the cryoprotective solution (pre-frozen semen) damage to the mitochondria, spermatic membrane and DNA fragmentation occurs as a consequence of the hyperosmotic shock and the toxicity of the cryoprotectant and that this damage increases during the freezing and thawing processes, which end up affecting the kinematics of the sperm and its fertilizing capacity. The cryogenic damage can result in decreased motility and fertility (Cabrita et al., 2014). In the present study, both in the dilution process with the cryoprotective solution and in the freezing/thawing process, all the variables that define sperm quality decreased in relation to what was observed in fresh semen.
The pre-frozen semen showed that the greater the cryoprotectant inclusion, the less total motility is observed; but when this variable is considered in the post-thawed semen, only the interaction between both factors was significant (p <0.015), obtaining the highest total motility for cryopreservation with 10% ETG, with no difference with 5 or 10% DMSO or DMA. According to this -although in the pre-freezing phase the greater the inclusion level, the greater the reduction of spermatic kinematics- in the post-thawed semen the effect of the cryoprotectant and inclusion level did not show difference in the percentage of rapid moving sperm, total progressivity, or VSL. This suggests that the cryoprotectants evaluated have differentiated effects in the pre-freezing phase, but, after the freezing/thawing process, no significant difference is observed in the quality of the thawed semen.
In all cases, total motility of the thawed semen was reduced to more than half than that observed in fresh semen (72.6%). The highest total motility for cryopreserved-thawed semen was obtained with 10% ETG (22.2%) and 5% DMA (19.0%). Reduction of sperm motility in the thawed semen is a consequence of the hyperosmotic shock between the cryoprotective solution and the semen during the pre-freezing phase and the damage caused during freezing and thawing (Padilla, 2014, Cabrita et al., 2014).
In the thawed semen, motility loss could be associated with mitochondrial damage as a consequence of oxidative stress due to the release of reactive oxygen species (ROS) (Bilodeau et al., 2000; Marti et al., 2008; Padilla 2014) and membrane damage (Cabrita et al., 2014). Sperm cells are sensitive to oxidative stress since their plasma membrane is rich in polyunsaturated fatty acids, making them vulnerable to ROS attacks (Fang et al., 2014). Some studies claim there are benefits in adding antioxidants in the cryoprotective solution to reduce the damage caused by oxidative stress and to protect the integrity of the sperm membrane and DNA during freezing and thawing (Dong et al., 2010; Butts et al., 2011). To minimize oxidative damage induced by ROS, a wide variety of antioxidants has been used in combination with basic cryoprotectants common to fish (Lahnsteiner et al., 2010; Hagedorn et al., 2012; Figueroa et al., 2018). The purpose of adding vitamin E (0.4%) in the present study was to reduce the oxidative effects.
Similar to the present study, low motility of thawed semen has been observed in other siluriformds, such as Clarias gariepinus (Viveiros et al., 2000), Pangasius gigas (Mongkonpunya et al., 2000) and Ictalurus furcatus (Lang et al., 2003). Good fertilities were observed by Viveiros (2011), although low motility was not reported; suggesting that even when it is immobile, alive sperm has fertilizing capacity.
In the present study, rapid sperm in the thawed semen were lower than 1.0% and medium velocity sperm did not exceed 5% in either case. According to Cabrita et al. (2014), decreases in sperm motility in the cryopreserved-thawed semen are due to the absence and degradation of fundamental proteins in intracellular signaling events required for motility. Using cryopreserved semen of white catfish with ETG between 6 and 10%, Padilla (2014) reported that mitochondria suffer damage since they come into contact with the cryoprotective solution (pre-freezing) and during the freezing and thawing processes, and mitochondrial damages are correlated the with the loss of motility in the thawed semen.
Linear velocity is closely related to fertility (Moore and Akhondi, 1996). In the present study, the highest VSL was observed with 5% ETG (8.6 ± 7.5 μm/s) and the highest VCL with 5% DMA (29.8 ± 7.0 μm/s); there was a reduction of approximately half the velocity after the cryopreservation process. This loss of velocity could be a consequence of the damage caused during cryopreservation, being only a small fraction of the viable sperm cells, reducing the fertilizing capacity of thawed semen (Figueroa et al., 2018). Motility loss in the thawed semen is associated with damage to the mitochondria as a consequence of oxidative stress due to the release of reactive oxygen species (ROS) (Cabrita et al., 2014; Padilla, 2014) affecting the mitochondrial respiratory efficiency (Ferramosca et al., 2013).
As a possible cause of mitochondrial damage, Yao et al. (2000) suggested the morphological changes occurring in the medium part of the sperm, which affect the dense envelope of the mitochondria. The loss of mitochondrial functionality reduces the membrane potential of the mitochondria, causing loss of motility and sperm velocity. Additionally, the velocity loss (VCL and VSL) occurring after freezing and thawing can be caused by damage to DNA integrity, which is possible when osmolarity and concentration of the cryoprotectants is not appropriate (Li et al., 2008).
According to Padilla (2014), the decrease of total motility, reduction of fast and medium velocity and increase of the slow and static sperm, as well as decrease in velocities (VSL, VCL) is a consequence of the damages suffered by the mitochondria, membranes and DNA during pre-freezing, freezing, and thawing.
In the present study, total progressivity in fresh semen (14.6 ± 11.0%) was greater than that obtained with cryopreserved-thawed semen. The highest total progressivity in cryopreserved-thawed semen was observed with 10% ETG (1.3 ± 1.9%); a reduction to one tenth in relation to that observed in fresh semen. Working with Acipenser fulvescens,Ciereszko et al. (1996), reported 35% progression at the time of activation and 26.2% five minutes later for cryopreserved-thawed semen (10% DMSO); values higher than those observed in the present study.
CONCLUSION
According to our results, DMSO, DMA and ETG included at 5 or 10%, combined with 6% glucose, skimmed milk powder and 0.4% vitamin E are viable cryoprotective solutions for semen cryopreservation of Pseudoplatystoma magdaleniatum, resulting in total mobility between 10 and 22%. However, fertility tests are suggested to determine the fertilizing capacity of semen frozen with these solutions.