INTRODUCTION
The economic importance of passion fruit (Passiflora eNulis cv flavicarpa Deneger) is given by its consumption as a fresh fruit, in the making of essences and make up, due to the content of vitamin C and minerals. These rea-sons have permitted its use as dietary complement (Cauz-Santos et al., 2017; Claro et al., 2018). In Colombia in 2016, 59.690 Ton of passion fruits were produced, distributed in 7888 ha; having Meta (1.513 ha), Antioquia (1.410 ha) and Huila (1.402 ha) as the main producing departments (Departamento de Administrativo Nacional de Estadística, 2016).
By 2050 in Colombia, significant increases of temperature, more erratic rainfall and higher prevalence of plagues and diseases are expected (Lau et al., 2011), it is estimated that temperature would increase 2.5 °C; with contrasting implications in different departments of the country.
Looking forward to attending these projections, it is necessary to develop climatically-intelligent crops, where vegetable genetic enhancements come as an alternative to obtain varieties with tolerance to limit factors such as stress for water deficit (Araus & Kefauver, 2018).
Passion fruit has been reported as a crop that does not tolerate water deficit (Carr, 2013) when changing the ecophysiology of the plant. Similarly, Fischer & Casierra-Posada, (2009) establish the needs of the plant for the conditions of Colombia in terms of altitudinal range, soils, weather and handling. It is a diploid plant (2n=18), non-self-compatible and requires of insects for its pollination (Munhoz et al., 2018), which reduces its genetic variability through time (Cerqueira-Silva et al., 2014).
In-vitro growing has been used to obtain plants resistant to drought, from somaclonal variants (SV) and in-vitro selection. These methods are based on obtaining and inducing genetic variation among the cells, tissues and/ or organs in the plants grown and regenerated (Rai et al., 2011). This strategy has been used in crops such as sorghum (Tsago, Andargie & Takele, 2014), sugar cane (Kumar, et al., 2014) wheat (Chachar et al., 2014), banana (Marssaro et al., 2017).
Polyethylene glycol (PEG), has been used in studies of tolerance mechanisms (Tsago et al., 2014), in the dis-crimination of genotypes (George, Jatoi, & Siddiqui, 2013), in the in-vitro selection of explants and SV (Verma et al., 2013), in the test of rice to water (Eslami et al., 2018), the goal of this research was to select in-vitro somaclonal variants (SV) of passion fruit (Passiflora eNulis var flavicarpa Deneger) tolerant to water deficit using polyethylene glycol.
MATERIALS AND METHODS
The study was carried out in the Plant Protection Laboratory - GEBIUT from University of Tolima (Ibagué, Colombia). Plant material was collected in 2014 in the Municipality of Dolores - Tolima (geographic coordinates 3°47' 3.64" N/ -75°02'74.8"W) in commercial plantations of passion fruit (Sistema de informacion sobre biodiversidad de Colombia, 2018). The material collected was introduced in the in-vitro germoplasm bank of the GEBIUT Laboratory.
The research was done in four stages as follows: Phase I: callogenic; Phase II: direct and indirect organogenic; Phase III: water stress induction, and Phase IV: in-vitro selection.
Phase I: genetic variability induction in three segments of foliar tissues (base, mid and apical), using the MS culture medium (Murashige & Skoog, 1962) with variations in the concentrations of 2,4-D (0, 1, 2 and 4 mg-L-1), planting 20 repetitions per each concentration and testing the percentage of callous generated by foliar segment during four weeks after planting.
Phase II indirect and direct organogenesis: Once the callous were induced, these were planted in an MS + BAP + ANA medium (relations 0.6:0.3 and 0.3:0.6) and MS + KINETIN + ANA (relations 0.2:0.7 and 0.7:0.7). As variable answers were obtained length, thickness, number of roots and stems as well as length, width, and number of leaves and wet weight of plants. These organogenic media were used in the stage of water stress.
Phase III: for water stress induction was used polyethylene glycol (PEG) 6000, used in in-vitro water deficit studies, using variations in the concentration of PEG (0,20, 25 and 30 g-L-1 equivalent 0; -0.05; -0.06 and -0.07 MPa of water retention according to García et al., (2015) and similar to the reported for Nath et al., 2004, over the best organogenic medium of phase. The length and thick-ness variables of roots, and length and width of leaves were used as morphometric markers; and as physicochemical markers were used the levels of chlorophyll using a CCM-300 Opti-Sciences® equipment, by reading the double wavelength at 700 nm - 710 nm and 730 nm -740 nm and total sugars using the Dubois, M., (1956) method, using glucose as pattern (1.01 mg mL) and absorbance reading at 490 nm in a BIO-RAD Smatspec 3000® spectrophotometer, in SV with a growth of 6 months and submitted to water stress for one month.
Phase IV: In vitro selection of plants with the best response against the water deficit generated by PEG. In this phase, the SV plants with the best performance in terms of morphometric measurements, and levels of chlorophyll and total sugars were selected.
Statistical Analysis: A completely random, experimental design was used; and to determine statistical significance of each of the variables measured, was carried out a one-way variance analysis for each phase of the test and per variable group, in case of showing significant differences (test F to 0.05), it was proceeded to make comparison tests of mean minimum significant difference (DMS) to 0.05.
RESULTS AND DISCUSSION
Phase I. Statistical differences were found among the concentrations of 2,4-D in the production of callous and no differences were found among the segments of foliar tissue in the production of callous. The first tissue to make callous was the base segment with petiole. The MS medium plus 2,4-D (4 mg-L-1) produced callous in less time (8 days) compared to the other treatments, but after four weeks, it was the treatment with the highest death rate of callous. MS treatment with 2,4-D (2 mg-L-1) produced a higher quantity of callous two weeks after planting and remained healthy after four weeks in 95% of the segments planted initially.
Out of 160 tissues with callous, only 3 generated indirect organogenesis, two of them stopped their growth and one had full formation with roots, stems and leaves. The regenerated callous was a base segment of foliar tissue coming from the MS + 2,4-D (2 mg-L-1) medium. Given the restrictions of vegetable material, this plant was used in massive multiplication until reaching 60 plants for the next phase.
Phase II. Variance analysis for the response variables of phase II showed significant differences among the treatments where the MS + ANA + BAP (0.3:0.6) medium showed the best averages to the root length (15.14 cm), stem (16.72 cm) and leaves (14.51 cm) and root thick-ness (0.76 cm) stem (1.25) and width of leaves (6.75) (Table 1).
Table 1 Averages of morphometric variables for in-vitro organogenic growth médiums of SV passion fruit (phase II)
| MEDIUM | ROOT | STEM | LEAVES | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LENCTH | THICKNESS | LENCTH | THICKNESS | LENCTH | VVIDTH | |||||||
| A+B (0.3:0.6) | 15.14 | BC | 0.76 | C | 16.72 | 5 | 1.25 | A | 14.51 | BC | 6.75 | BC |
| A+B (0.6:0.3) | S.34 | AB | 0.63 | BC | '3.-6 | A | 1.09 | A | 14.19 | BC | 7.43 | C |
| K+A (0.2:0.7) | 20.59 | C | 0.53 | AB | 14.68 | AB | 1.10 | A | 12.35 | B | 6.25 | B |
| K+A 10.7:0.7. | -2.23 | \ | 0.23 | A | 10.64 | A | 1.03 | A | 9.03 | A | 4.26 | A |
A= Naphtalene acetic Acid. B= Benzylaminopurine. K=Kinetin.
Averages with the same letter do not show significant differences (DMS 0.05)
These results differ from the ones found by Medina et al., (2014) which report that the mix ANA+BAP, did not stimulate radicular growth in tobacco seedlings.
The increase of thickness in roots, also known as secondary growth, is stimulated by the right combination of auxins and cytokinins. This behavior is described as well by Schaller, Bishopp & Kieber, (2015). Statistical differences were found in the stem length and leaf width variables. These results match what was found by Manjarrés-Hernández & Perea-Dallos, (2012) in gulupa, a lesser passion fruit (Passiflora edulis Sims).
Phase III. Morphometric measurements of SV roots put under water stress with PEG 6000 in the four concentrations showed significant differences, concentration mediums of PEG 20 and 25 g-L-1 had the best results in the increase of root thickness with 0.69 and 0.58 cm respectively. Seedlings responded to water stress increasing root thickness, except in the control treatment (0,36 cm ) and the mediums with the highest concentration of PEG 30 g-L-1 0.52 cm (Table 2 and Figure 1). similar results are reported by Albiski et al., (2012) in potato, where in a 4% concentration of stressing agent, some seedlings in-creased length and thickness of roots. The treatment with the least influence in the increase of root thickness of passion fruit SV corresponded to the control.
Table 2 Development averages of some vegetable organs in passion fruit SV in function of PEG concentration used as agent of water déficit. Averages with the same letter do not show significant differences (DMS 0.05).
| PEG | ROOT | STEM | LE4VES | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LENCTH | THICKNESS | LENCTH | THICKNESS | LENCTH | VVIDTH | |||||||
| 0 | 8.30 | AB | 0.36 | A | 12.67 | A | 1.16 | A | 9.71 | A | 5.32 | A |
| 20 | 18.16 | B | 0.69 | B | 15.64 | A | 1.19 | A | 15.35 | B | 7.19 | B |
| 25 | 4.01 | A | 0.58 | B | 13.33 | A | 1.13 | A | 14.51 | B | 7.35 | B |
| 30 | 10.87 | AB | 0.52 | AB | 13.55 | A | 1.04 | A | 10.51 | A | 4.83 | A |

Figure 1 Longitudinal growth comparison of passion fruit SV roots in four concentration of root PEG and organogenics médiums, notice root growth and thickness concerning the control. A. Control treatment, B, C and D, Organogenic médium with PEG 6000 in concentrations of (0, 20, 25 and 30 g/l).
Influence of PEG in the size of leaves of passion fruit SV showed significant differences, where the treatment with the highest concentration (30 g-L-1) showed the least leaf width (Table 2). The highest width was found in the con-centration of 25 g-L-1 and similar to 20 g-L-1. This shows that PEG at 30 g-L-1, generated negative impacts in tis-sues, especially on leaf growth, similar to what was reported by Hassan et al., 2004, Albiski et al., (2012) and Loaiza & Mesa (2019). As for the number of leaves, it is observed that the treatment of PEG 20 g-L-1 was where the highest amount of leaves was generated, having statistically significant differences with the remaining treatments (Figure 2).

Figure 2 Comparison of leaves produced under water stress in passion fruit SV, notice the growth of leaves in PEG 20 y 25 g-L"1 treatment. A. Control treatment, B, C and D, Organogenic médium with PEG 6000 in concentrations of (0, 20, 25 and 30 g/l).
Chlorophyll (F=6.89**) and total sugar levels (F=59.90**) in the SV according to the concentration of PEG showed highly significant differences. Highest chlorophyll contents and sugars were recorded newly to the 25VS1 seedling in treatment PEG 25 g-L-1, Table 3 and figures 2 and 3.
Table 3 Chlorophyll levels and Total Sugar Levels in Somaclonal variants of P. edulis in medium with different concentrations of PEG.
| Seedling | Total Sugars (mg Equívalent to glucose/g dry vegetable) | Chlorophyll (mg m2) | ||
|---|---|---|---|---|
| 25VS1 | 71.55 | G | 425.35 | E |
| 25T2 | 17.09 | BCD | 187.35 | CD |
| 0VS2 | 30.56 | E | 326.68 | DE |
| 0VS1 | 18.28 | CD | 310.02 | DE |
| 20T2 | 41.87 | F | 320.68 | DE |
| 30T2 | 11.12 | ABC | 231.68 | CDE |
| 25VS2 | 7.57 | AB | 209.02 | CD |
| 30T1 | 20.58 | DE | 139.35 | CD |
| OTESTIGO | 14.52 | BCD | 185.35 | CD |
| 0VS3 | 14.33 | ABCD | 215.24 | CD |
| 0VS4 | 14.52 | BCD | 185.35 | CD |
| 20T4 | 14.52 | BCD | 185.35 | CD |
| 20VS3 | 14.52 | BCD | 185.35 | CD |
| 25T4 | 14.52 | BCD | 185.35 | CD |
| 25VS3 | 14.52 | BCD | 185.35 | CD |
| 30T4 | 14.52 | BCD | 185.35 | CD |
| 30VS3 | 14.52 | BCD | 185.35 | CD |
| 25T3 | 18.42 | CD | 115.35 | ABCD |
| 20VS1 | 17.69 | BCD | -32.32 | AB |
| 30VS1 | 8.50 | ABC | 155.35 | BCD |
| 30T3 | 9.13 | ABC | 181.35 | BCD |
| 25T1 | 14.65 | BCD | 1.35 | AB |
| 20T1 | 6.15 | A | 176.68 | BCD |
| 30VS2 | 10.73 | ABC | -79.65 | A |
| 20YS2 | 11.41 | ABCD | -43.65 | AB |
| 20T3 | 8.08 | ABC | 60.35 | ABC |
Averages with the same letter do not show significant differences (DMS 0,05)
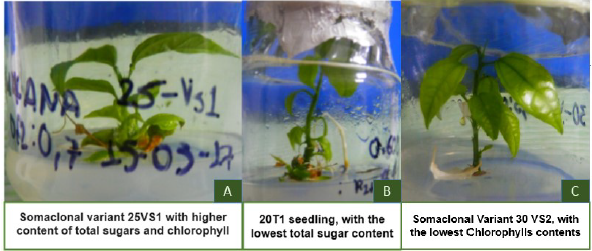
Figure 3 Somaclonal variants of passion fruit. A. SV 25VS1 with the highest total sugars and Chlorophyll con-tents. B. 20T1 lowest total sugar contents, and C. VS 30VS2 lowest chlorophyll contents respectively.
Phase IV. For phase IV, the contents of total sugars and chlorophyll levels in SV seedlings showed significant differences. It was found that the highest content of total sugars and chlorophyll levels were in SV 25VS1 with 71.55 mg equivalent to glucose/g dry vegetable and 425.35 mg m-2 (table 3 and figure 3), which corresponds to the first SV planted in the culture medium MS + ANA + BAP + PEG 25 g-L-1, a medium with high levels of water retention. Similar results are reported by Moreno-Bermúdez et al., (2017), who found high levels of chlorophyll in in-vitro banana seedlings submitted to water deficit using PEG (Table 3).
In contrast, plant 25VS2, despite coming from the same callous of seedling 25VS1, showeds the lowest levels of total sugars with 7.57 mg, which would indicate genetic variability of the materials planted and exposed to the stressing agent, this is a promising plant for possible plant breeding programs at the University of Tolima.
The lowest level of chlorophyll was found in the seedling 30VS2 (-79.65 mg m2), corresponding to the second SV group planted in the stressing medium containing PEG 30 g-L-1, the highest concentration of the stressing agent (Table 3).
The increase of total sugar levels against the water stress produced by the PEG has been explained by Herrera et al., (2012). It is also important to highlight other materials such as 30T1 (20.58), 0VS2 (30.56) and 20T2 (41.87), which showed high total sugar levels, indicating tolerance to water stress; 30T1 and 20T2 are seedlings that did not regenerate from callous. This behavior may be due to SV inducted by factors such as age of tissues and number of sub-cultures (Sánchez Chiang & Jiménez, 2008).
0VS2 corresponds to the second SV planted in a medium without PEG, Naser et al., (2010) and (Díaz V, Lazo R, Portilla Ll, Ponce-Soto, & Marangoni, 2012), report a generalized increase of the contents of the total sugars. This is because soluble sugar content tends to remain on the leaves of the plants. The increase in sugar content soluble by inversion of some carbohydrates can contribute to improve tolerance to dissection and allows keeping metabolic activity (Vijayakumari & Puthur, 2016).
High total sugar contents in plants submitted to water stress would indicate an adaptation response by the plant, similar to chlorophyll contents. Seedlings increase photosynthetic pigments when stressed in order to compensate water deficit. These results concur with the ones reported by Díaz Valencia, (2016), which states that in the plants, at cellular level, happens an osmotic adjustment that aims to keep the osmotic potential during the period of stress using sugar or other compatible solutes (Castañeda-Castro, Gómez-Merino, Trejo-Téllez, & Pastelín-Solano, 2015) and by accumulation of ions, particularly K+ (Ma, Wang, Wang, Zhong, & Cheng, 2019).
CONCLUSIONS
The MS medium with 2,4-D (2 mg-L-1), there was a higher quantity of callous (95%), despite take more time in generation, it was the treatment that showed a higher survival rate of tissues.
It was possible to obtain somaclonal variants of passion fruit from only one regenerated seedling; coming from the callous of a base segment of foliage tissue, originated in the callogenic medium MS + 2,4-D (2 mg-L-1). The organogenic medium MS + ANA + BAP (0.3:0.6 mg-L-1) allowed plant regeneration, which shows the difficult obtainment of indirect organogenesis from callous of passion fruit foliar tissue.
The best results concerning morphometric measurements were obtained from the culture mediums containing PEG 20 and 25 g-L-1. Chlorophyll and total sugar levels in the SV showed significant statistical differences. The highest contents of chlorophyll and total sugar levels were recorded in an SV planted in a medium with PEG 25 g-L-1, a medium with high levels of water retention.
The result described above shows that it was possible to obtain genetic variability by callogenesis induction and later indirect and direct organogenesis, supported with the morphometric and biochemical measurements taken on the different tissues.
The results obtained are important raw materials to continue with the processes of phenotypic and genotypic selection of passion fruit seedlings that show different degrees of tolerance to water stress, aiming to a genetic improvement process of passion fruit in Universidad del Tolima.














