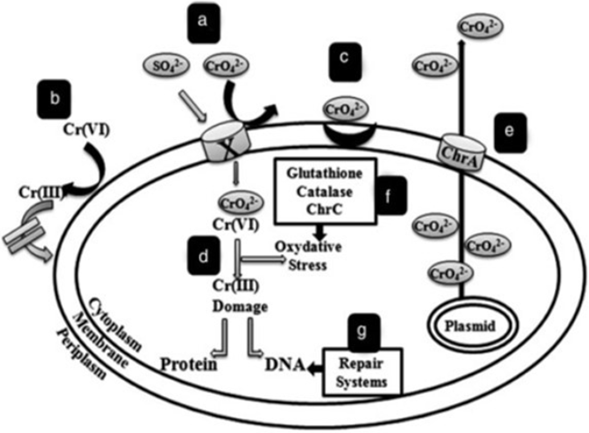
INTRODUCTION
Chromium is a heavy metal that can be found in six oxidation states in nature, being the Cr+3 and Cr+6 species the most stable ones, known as trivalent (Cr3+) and hexavalent (Cr6+). The Cr3+ form is considered essential and is involved in the metabolism of glucose and insulin, regulating cholesterol and triglyceride levels (Higdon, Drake, and Delage, 2003). However, some studies indicate that, under certain conditions, Cr3+ can cause genomic instability and it has been suggested that it is not essential, since it does not participate in the structural stabilization of enzymes or in nutrient uptake (Eastmond, MacGregor, and Slesinski, 2008; Wise and Wise, 2012). From the chemical point of view, Cr3+ is poorly soluble and of low mobility when it is complexed with the organic matter of the soil (Gutiérrez Corona et al., 2010). The Cr6+ form is mobile, soluble, and permeable through the cell membrane, causing DNA and some protein damage, therefore considered as mutagenic, teratogenic, and carcinogenic. In addition, it is reported to cause skin damage when a direct contact is presented, to induce respiratory tract cancer when inhaled, and if ingested, it can cause stomach ulcers with complications that could lead to death (Horel, 2017; Nayak, 2017).
Different studies have been carried out on bacteria isolated from chromium-contaminated matrices in order to stabilize or capture the metal. Bacillus cereus is a cosmopolitan bacterium of environmental interest and has been investigated in the health and food industries (Banerjee and Ghoshal, 2016; Iranzo, et al., 2018; Sánchez, et al., 2016). This bacterial species has been reported to present the ability to reduce Cr6+. In 2009, in Medellín (Colombia) B. cereus was obtained (among other bacteria of the Pseudomonas genus) from runoff biofilms in a tannery industry. It was found that it reduced Cr6+ by 99.8% to an initial concentration of 28 ppm in 100 h (Martínez Yepes, 2009). In 2016, B. cereus was isolated from electroplating wastewater in Cali (Colombia), in a concentration of 1 0 ppm of Cr6+, accomplishing a reduction of 100% in 10 h (Mora Collazos, 2016). This same bacterium has been also reported to be tolerant to variations in pH, temperature, salinity, and other conditions, which could support its use in the bioremediation field, due to its capacity for resilience and adaptation to adverse conditions (Singh, et al., 2013).
The Ochrobactrum anthropi has been identified as a nosocomial pathogen, causing infections that are difficult to treat, due to its resistance to most antibiotic principles (Chudasama and Thaker, 2017; Haviari, et al., 2016; Henderson, et al., 2016). However, its potential in biore-mediation has exceeded expectations, attesting its ability to metabolize different aromatic compounds and hydrocarbons, as a source of carbon and energy (Chudasama and Thaker, 2017). It has also been reported as an alternative in fuel-related research, due to its ability to produce biosurfactants (Ibrahim, 2011). Some authors report reductions of Cr6+ in concentrations of 200 ppm, from 95 to 100% in 24 h on a dead biomass (Cheng, et al., 2010; Francisco, et al., 2002).
The Staphylococcus saprophyticus has been reported in clinical cases in humans mainly associated to urinary tract infections, with few studies in the environmental area. However, it has the capacity to tolerate Cr6+ in concentrations up to 3,000 ppm (Alekhya and Subbaiah, 2016), without reducing it.
This work aimed to evaluate the chromium reduction capacity by bacteria isolated from a biosolids matrix obtained at the San Fernando Wastewater Treatment Plant (WWTP), located in Medellín (Colombia).
Materials and methods
Isolation of bacteria from biosolids
Biosolids samples were randomly collected from the dehydrators of the San Fernando WWTP, located on the South of the city of Medellín, Province of Antioquia (Colombia). A physicochemical characterization of the samples was performed. The concentration of heavy metals was determined by the atomic emission method -using Inductively Coupled Plasma (ICP), and the cold vapor method was used to determine chromium. The critical values -regulated by the Decree #1287 of 2014 of the Ministry of Housing, City, and Territory of Colombia (in Spanish, Ministerio de Vivienda Ciudad y Territorio), were considered as a reference (1,200 mg K-1). Eight aliquots were randomly collected (for a total of 10 g of wet base) and suspended in 100 ml of 0.1% w/v sterile peptone water for 15 min at 15 psi and pH of 5.5. This suspension was stirred for 1 h, and then, 100 were used for culture on a nutritive agar enriched with Cr6+ (K2Cr2O7) at 400, 500, 600, 800, and 1000 ppm, and three replications each, using the surface exhaustion method. Culture media were incubated at 35°C for 7 days (US-EPA, 1992). Bacterial colonies were obtained from a nutrient agar under the same incubation conditions. The morphological of the isolates was based on their shape, size, consistency, brilliance, translucency, among others features. Isolates were then cryopreserved at -20°C on a nutrient broth with 20% v/v glycerol.
Determination of the bacterial capacity to reduce Cr 6+ The cryopreserved isolates were directly activated on a nutrient agar and incubated at 37°C for 40 h. Subsequently, three colonies were transferred to a Luria Bertani (LB) broth, enriched with 1 00 ppm of Cr6+, and then incubated at 37°C for 48 h. Aliquots of 1.5 mL were taken at 0, 24, and 48 h of culture. The samples were centrifuged at 10,000 centrifugal force (g) for 20 min, and the supernatant was separated and refrigerated at 4°C to perform the chromium measurements at the end of the assay. Simultaneously, one the samples was incubated for 96 h following the same protocol detailed above. The final Cr6+ concentration was determined by the diphenylcarbazide colorimetric method, following EPA protocol 7196A. From a stock of 100 ppm of Cr6+, serial dilutions were made until a concentration of 1 ppm was reached. The volume of the EPA 7196A method reaction mixture was changed to 9.5 mL of diluted sample, 0.1 mL of 1 N sulfuric acid, with 0.2 mL of a diphenylcarbazide solution (250 mg of 1.5 diphenylcarbazide in 50 mL of acetone) and completed with distilled water to a final volume of 10 mL. Ten minutes later, the absorbance was determined at a wavelength of 540 nm. For the estimation of the Cr6+ concentrations of the samples, a calibration curve was prepared using 0.25, 0.5, 1, and 2-ppm concentration solutions (US EPA, OSWER, ORCR, 1992).
Total chromium measurement
Samples collected at 0, 24, 48, and 96 h, with Cr6+ concentrations of 100 ppm diluted until 1 ppm, and a pH reduced to a value of 1.0 (with nitric acid 0.1 mL at 65%, v/v), were subjected to total chromium measurement, using an Agilent Technologies 4100 MP-AES atomic emission equipment. Data processing was carried out with the MP Expert program (vers.1.5.1.6821).
Determination of the microbial growth curve in the presence of Cr 6+
A growth curve was performed by spectrophotometry. The LB-bacteria were inoculated on a 96-well ELISA dish containing 100 ppm of Cr6+ for 48 h at 37°C. For the identification of the bacterial isolates, the absorbance at 600 nm was determined every hour (after shaking for 30 seconds), using a Multiskan go TM spectrophotometer, Thermo SCIENTIFIC.
Identification of bacterial isolates
The isolates obtained were biochemically identified using the Biolog Microstation ID System OmniLog®, this system provides 96 microplates which have different means for the biochemical characterization of each strain, the appendix 1- 4 show the biochemical rection made in each well in the microplate; the equipment provides a specific kit for the bacteria cultivation and distribution among the wells. The bacteria were cultivated in the BUG agar, after 24 h and distributed in the 96 wells equally and finally taken to the equipment to do the measures, the results for the bacteria grow and the biochemical reactions it takes 24 h.
Statistical analysis
Differences on the ability of the bacterial strains to reduce Cr6+ at different concentrations were estimated. Normality and homogeneity estimations were performed using the Shapiro Wilk (Chacon Montalvan, 2014), and Bartlett tests (Mellado, 2013), respectively. Then, a one-way analysis of variance (ANOVA) was used to determine whether there were any statistically significant differences between the means (Sokal and Rohlf, 1995). Finally, treatment averages were analyzed by the Tukey's honestly significant difference (HDS) and multiple comparisons tests, using the R-student software (R-core team 2017).
RESULTS
Bacterial isolates and Bacterial identification
Thirty-two bacterial biotypes, from seven different species, were obtained from media with concentrations of 400 to 600 ppm of Cr6+). The three strains of greatest interest were O. anthropic, B. cereus and S. saprophyticus. In addition, four other strains of bacilli were found with some interest to remove chromium, Bacillus megaterium, Bacillus subtilis, Bacillus firmus, Bacillus lentus.
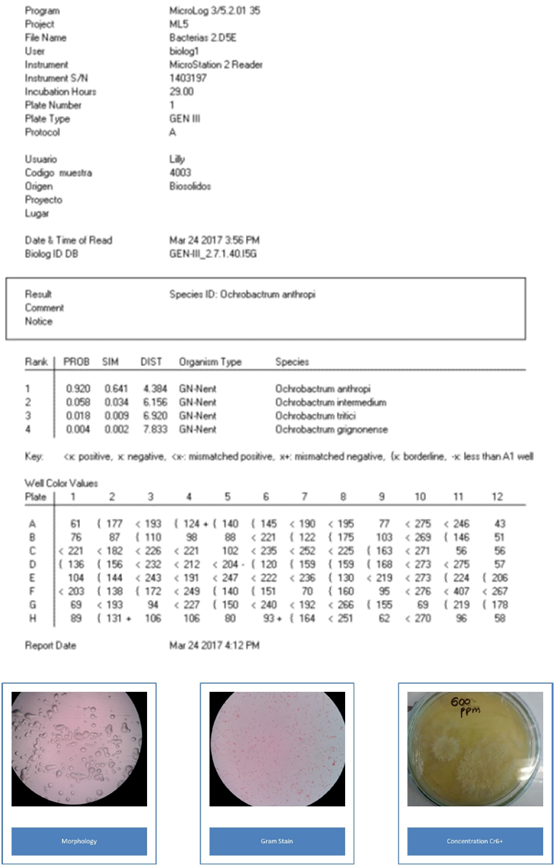
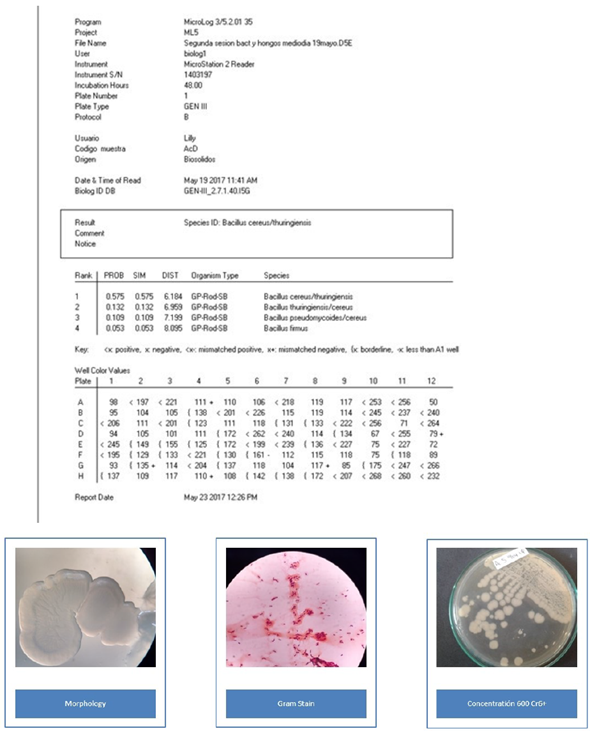
The morphological identification of the colonies allowed the separation of microorganisms such as Gram positive and Gram-negative, bacilli and cocci. S. saprophyticus is a creamy, rounded, yellow and smooth-edged spore, Gram positive cocci. B. cereus is a Gram-positive bacillus with round, white rough, dry consistency spores. O. anthropic is a Gram-negative bacillus, round and smooth, with a juicy texture and a translucent white color. The characterization was carried out with Biolog Microstation ID System OmniLog®. The values show each biochemical reaction and the isolated name with its respective probability.
Hexavalent chromium-reduction assays
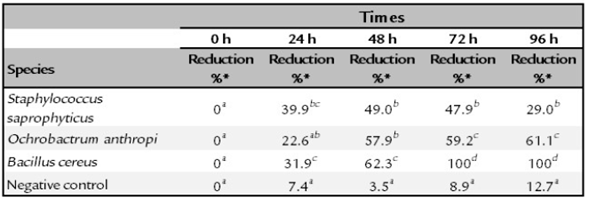
The O. antrhopi was the most tolerant to the metal, showing growth in concentrations of 300 to 600 ppm of Cr6+, and presenting the highest reduction (63% at 48 h), followed by B. cereus and S. saprophyticus with 62% and 49%, respectively. Figure 1 shows the bacterial behavior on the reduction of total chromium during the test carried out during 48 h (without significant changes for the three bacterial species of the study). During the first 24 h, the curve reached its lowest point, obtaining a significant decrease of 58.2% on the total chromium. Figures 2 and 3 show the Cr6+ reduction kinetics of the three mentioned bacterial strains and a negative control, during 48 and 96 h, respectively.
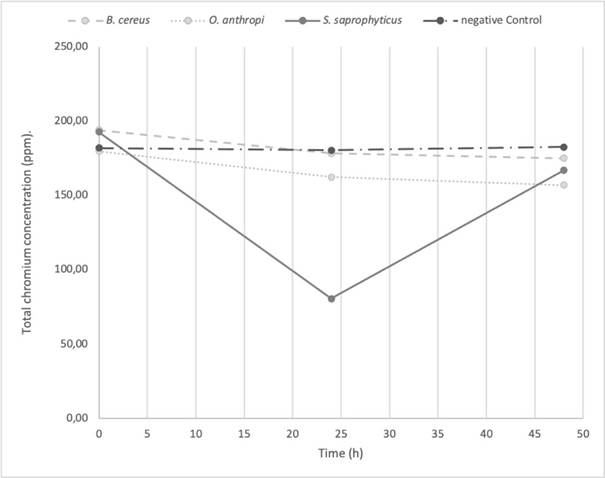
Figure 1 Total chromium reduction kinetics at 48 h, pH 5.5 by Ochrobactrum anthropi, Staphylococcus saprophyticus, Bacillus cereus species and Negative Control. Three replications each, obtained from biosolids of the San Fernando Wastewater Treatment Plant (WWTP), in Medellin (Colombia)
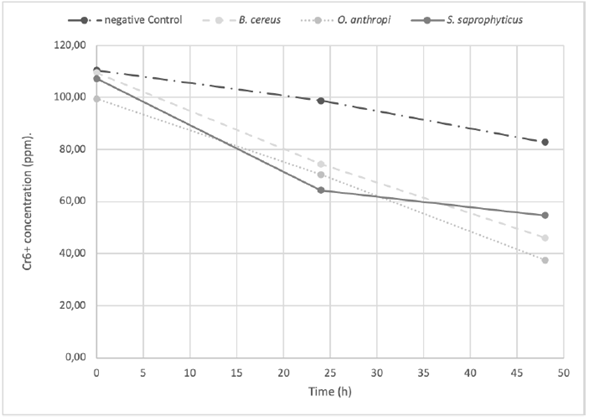
Figure 2 The Cr6+ reduction kinetics at 48 h, pH 5.5 by Ochrobactrum anthropi, Staphylococcus saprophyticus, Bacillus cereus species and Negative Control. Three replications each, obtained from biosolids of the San Fernando Wastewater Treatment Plant (WWTP), in Medellin (Colombia).
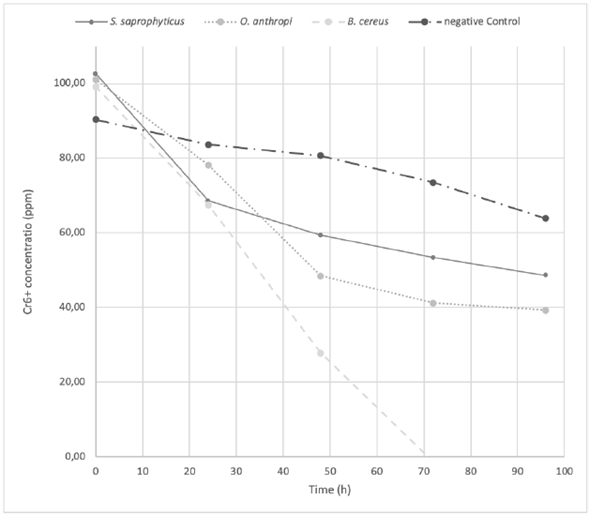
Figure 3 The Cr 6+ reduction kinetics at 96 h, pH 5.5 by Ochrobactrum anthropi, Staphylococcus saprophyticus, Bacillus cereus species and Negative Control. Three replications each, obtained from biosolids of the San Fernando Wastewater Treatment Plant (WWTP), in Medellin (Colombia).
Growth in the presence of Cr 6+
Figure 4 shows the bacterial growth in cultures enriched with Cr6+. A higher absorbance reflects a greater growth of the colonies as a function of time. At 48 h, B. cereus and O. anthropi did not completed their exponential growth phase, while S. saprophyticus reached it around 24 h later.
Statistical analysis
Normal and homogeneous results were obtained [W = 0.82641 (p = 0.01901); Chi square = [15.318 (p = 0.001564)]. Table 1 presents a summary of the behavior and the percentage reduction of Cr6+ from 0 to 96 h. For the initial time (0), all the treatments were equal; therefore, there were no differences between them. At 24 h, differences were observed, although there was a tendency to present common results among microorganisms. At 48 h, the only strain that showed a significant difference was B. cereus; however, at 72 and 96 h, all strains showed different reduction levels of Cr6+.
DISCUSSION
In the present study, different Cr6+tolerant bacteria (in concentrations of 100 to 600 ppm) were isolated under standard aerobic culture conditions, in a nutrient medium enriched with this metal. Among the isolates obtained, O. anthropic, B. cereus, and S. saprophyticus showed the greatest ability to reduce and tolerate Cr6+.
Treatments on O. anthropi and B. cereus did not achieve the maximum reduction of Cr6+ at 48 h, as recorded by the growth curve of these two strains (Figure 4), since both strains were in their exponential phase at that time. Actually, as O. anthropi continued its exponential phase at 48 h, B. cereus was just starting this phase. This is the reason why it was considered to extend the chromium reduction curve up to 96 h (Figure 3). The S. saprophyticus strain showed a different behavior, since it completed its exponential growth phase in the first 24 h, reaching its stationary phase at 48 h and finally yielding cell death. Nevertheless, significant changes in the decrease of total chromium were not observed, remaining stable over time for all the strains. In all three cases, the growth behavior at 48 h was directly related to the reduction of the metal. Como podría explicarse esta observación, qué indicaría, que implicaciones tiene?
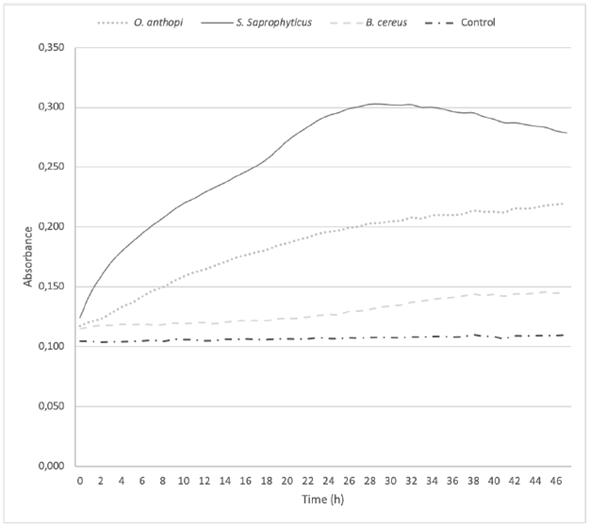
Figure 4 Bacterial growth of Ochrobactrum anthropi, Staphylococcus saprophyticus, Bacillus cereus species, and Negative Control, cultured in a Cr6+enriched media. Three replications each, obtained from biosolids of the San Fernando Wastewater Treatment Plant (WWTP), in Medellin.
Ochrobactrum anthropi presents high resistance to most families of antibiotics (Haviari, et al., 2014; Henderson, et al., 2017). However, studies on its potential in biore-mediation exceed expectations, as its ability to reduce different aromatic compounds and hydrocarbons in order to use them as a source of carbon and energy has been proven (Chudasama and Thaker, 2017). Its utility in the alternative fuels field has been also reported, due to its ability to produce biosurfactants (Ibrahim, 2011). Previous studies have found that this bacterium can reduce Cr6+ in concentrations of up to 200 ppm of chromium in a 24 h period from 95 to 100%, using their dead biomass (Cheng, et al., 2011; Francisco, et al., 2002). To the date, no reports on the use of the live biomass of this bacterium to reduce the metal are available, and, although in the present study the Cr6+ reduction was of 61% at 96 h, the reduced chromium concentration remained stable over time.
Bacillus cereus was the only strain that presented a total reduction of Cr6+ at 72 h (Figure 3). During this time, the hexavalent chromium became trivalent, remaining so until the end of the test; however, there was no decrease of the total chromium since the reduction percentage at 96 h was only 7%. This result reflects a clearly reducing behavior of the bacillus. Other authors have reported B. cereus as a cosmopolitan bacterium, used in bioremediation in the environmental research field, as well as in the health and food industry (Iranzo, 2018; Sánchez, et al., 2016; Banerjee and Ghoshal, 2017). Among the isolated bacteria, B. cereus showed the highest Cr6+ reducing capacity. In 2009, B. cereus was isolated from the runoff biofilm in a tannery industry in Medellín (Colombia). The strain reduced Cr6+ by 99.8% from an initial concentration of 28 ppm, over 100 h (Martínez Yepes, 2009). A study made by Mora Collazos, et al. (2016), reported the isolation of a B. cereus strain from wastewater in an electroplating company, with an initial concentration of 10 ppm of Cr6+, achieving a reduction of chromium of 100% in 10 h. Recent studies found an increase in the ability of B. cereus in the presence of Mn (II) and Mg (II) to reduce and remove hexavalent chromium to levels of 64% in 120 h. (Xu, et al., 2011)
Comparing the results obtained in this study, B. cereus showed a greater tolerance to the metal, growing at concentrations of up to 300 ppm of Cr6+ and achieving a reduction of the same in concentrations higher than those reported in the aforementioned studies. Other authors have reported that this species requires approximately 20 h to start growing in a culture medium enriched with more than 100 ppm of Cr6+ (Singh, et al., 2013). Figure 4 confirms that growth starts after 20 h in a culture with 100 ppm of Cr6+. Likewise, once growth begins, the chromium reduction rate accelerates, achieving reductions of Cr6+ to Cr3+ of 100% at 72 h in a shake culture. Bacillus cereus has been also reported as tolerant to variations in pH, temperature, salinity, and other conditions, which supports its use in the field as bioremediation due to its adaptability (Singh, et al., 2013).
Staphylococcus saprophyticus is an environmental bacterium, but has been also reported in clinical cases, mainly associated with urinary tract infections. It has the ability to tolerate Cr6+ in concentrations up to 3,000 ppm but without the ability to reduce it (Iyengar and Subbaiah Usha, 2016). The isolated strain reached its maximum reduction of Cr6+ from 39.9% at 24 h to 49. % at 48 h (Figure 2). The total chromium concentration over time showed an interesting behavior, since at 24 h the concentration decreased to 58.2%, but at the end of the test (48 h) the metal capture dropped to 13.5%, suggesting release of the initially captured metal.
Tahri, et al. (2011), reported that a microbiological cell presents different mechanisms to reduce Cr6+ to Cr3+, including the extracellular ability to reduce Cr6+ to Cr3+ using functional groups present on the cell surface; reduction in the cell membrane, usually preceded by the adsorption of Cr6+ to functional groups located on the bacterial cell surface; intracellular reduction of Cr6+, that when reduced to Cr3+, is released from the cell, then conserving a low cytoplasmic concentration of Cr6+, also facilitating the accumulation of chromate from the extracellular medium into the cell. (Appendix 5).
In the present study, the strain of S. saprophyticus captured, reduced, and then released the metal to the external medium.
Other species isolated during the research belong to the group of sporulated bacilli such as B. megaterium, B. subtilis, B. firmus, and B. lentus, but they did not present significant reductions of Cr6+ in comparison to the three strains already mentioned. However, literature report these bacteria as tolerant and Cr6+ reducers, as in the case of B. subtilis and B. megaterium isolated from wastewater associated with tanneries (Pan, et al., 2014; Martínez Yepes, 2009). In this specific report, these bacteria presented values between 22 and 35% of Cr6+ reduction, although, in the aforementioned reports the reductions exceeded these values.
CONCLUSION
From biosolids of a WWTP, different species of bacteria of interest in chromium bioremediation were isolated, among other microorganisms such as fungi and yeasts. Isolated bacteria showed high adaptability and ability to reduce Cr6+. The O. anthropic strain showed the greatest tolerance and Cr6+ reduction capacity. S. saprophyticus is a bacterium with the capacity to capture chromium and retain it for a considerable time, allowing its possible use in the design of biofilters for the purpose of co-remediation of waters contaminated with this heavy metal.
Bacillus cereus was the only strain capable of reducing the metal by 100% from its hexavalent form to the trivalent one, indicating its biotechnological potential and the possibility of being considered for environmental bioremediation programs. New concentrations of Cr6+ should be evaluated to observe their behavior and tolerance thresholds.