INTRODUCTION
Arbuscular mycorrhizal (AM) fungi are soil fungi that occur worldwide forming symbiotic associations with most plant families. Their importance in natural and semi -natural ecosystems is commonly accepted and characterized by improved plant growing as well as, an increasing plant resistance against biotic and abiotic stresses (Agnolucci et al., 2020). In Agricultural ecosystems under less favorite growing conditions, they play an important role by enhancing productivity and sustainability (Begum et al., 2019).
The plant-AMF interaction studies are difficult due to the obligate symbiont condition of the fungi (Silvani et al., 2019). AMF cannot complete their life cycle neither grow in axenic conditions without establishing a functional symbiosis with host plants (Srinivasan et al., 2014). However, in vitro root cultivation techniques, as a simplified system for the establishment of the arbuscular mycorrhizal symbiosis, have been widely used and it has shed new light on their molecular biology, cytology, genetics, physiology, systematics, and phylogeny (Danesh and Tufenkci, 2017).
The most obvious advantage of in vitro cultivation system is the absence of undesirable microorganisms, which makes it more suitable for large-scale production of high-quality inoculum (Ijdo et al., 2011; Danesh and Tufenkci, 2017) and for construct in vitro culture collections. Several AM fungi species had been cultured in root organ cultures (ROC) systems. Based on scientific literature and culture collections, it is estimated that over 100 different strains are maintained in vitro (Ijdo et al., 2011). However, there is evidence that many species are not able to germinate or colonize transformed roots under these conditions. Therefore, it is a challenge for many researchers to improve in vitro system by taking into account culture media composition, grows conditions, propagules and AM hosts.
Although, in Cuba much progress is made in the cultivation and reproduction of AMF as well as in the production of AMF inoculum, there is less experience with in vitro culture conditions. Constructing an in vitro collection of Cuban AMF strains would be an important achievement not only for research on plant -AMF interaction but also for building up an indigenous collection for agriculture use.
The main objective of this study was to establish and characterize an in vitro culture of Rhizophagus irregularis INCAM11 based on transformed chicory (Cichorium intybus L.) hairy roots.
MATERIAL AND METHODS
Biological Material
The strain INCAM 11, DAOM 711363 Rhizophagus irregularis (Walker & Schüßler) belongs to the AMF collection of the 'Instituto Nacional de Ciencias Agrícolas, (INCA)' from Cuba. Fungal spores were obtained from pot culture by using the wet sieving and decanting (Gerdemann and Nicolson, 1963) technique followed by sucrose centrifugation (Jenkins, 1964). Isolation was done under a dissecting microscope (Novel) at 10 -50 X magnification. Spores' clusters were stored at 4°C until their surface disinfection. The Ri-t-DNA transformed chicory (Cichorium intybus L.) hairy roots, supplied by Montreal University, Canada, were used to establish the in vitro root-organ culture.
Spores' disinfection procedure
INCAM 11 spores were surface disinfected according to a modified methodology of Cranenbrouck et al. (2005). Among 150 and 200 healthy young spores of INCAM 11 were transferred to the filtration membrane (0.44 µm), rinsed 3 times with sterile distilled water, and treated with Chloramin T 2% (with 2 drops of Tween 20) for 10 min. Then spores were washed 3 times with sterile distillated water and treated 10 min with antibiotic solution containing Streptomycin sulphate (0.02 %) and Gentamycin sulphate (0.01 %). The solution was filter on a disinfection apparatus through a sterile millipore filter (type HA, diameter 4.0 cm, pore 0.22 µm). Afterward, the membrane supporting the disinfected spores was gently transferred into plastic Petri plates (90 mm diam.) containing 20 mL of antibiotics solution and kept in the plates for 24 hours.
Monoxenic culture of INCAM 11 by root organ culture (ROC) Thirty INCAM 11 surface disinfected spores associated in clusters and Ri T-DNA transformed chicory hairy roots of 2 cm length were transferred to plastic petri plates (90 mm diam.) on a Modified Strullu and Romand (MSR) medium (Cranenbrouck et al., 2005). Forty plates were set up and incubated on inverted position at 27°C in the dark until the hyphae colonized the young roots, the mycelium dispersed profusely into the medium and new spores were produced.
Plates were checked every 48 h for spore germination and hyphal growth. First contact between fungus and host roots, new spore formation and extraradical hyphal length were recorded. Evaluations were carrying out under a dissecting microscope (Novel) at 10-50X magnification. A grid of lines was marked on the bottom of each Petri plate for the extraradical hyphal length and new spore formation. Hyphal length was monitored weekly during 3 months and spore's numbers were determined at the end of the experiment, following the methodology described by Voets et al. (2005).
The newly produced spores were sub-cultivated on fresh medium to confirm if they were able to form associations with a new in vitro host and complete their life cycle. Forty spores from the in vitro culture associated in clusters were extracted. Small pieces of medium containing spores were gently removed with a scalpel and located in the vicinity of fresh roots apex of 10 days of culture growing in new MSR medium. Plates were sealed with parafilm and incubated at 27 °C in the dark.
Isolation and molecular identification of associated microorganism
The most frequent associated microorganism colony to the in vitro culture of INCAM 11 was isolated and molecularly identified. When bacterial growth around fungus were observed, individual colonies were isolated on Nutrient Agar (Wright, 1934) medium and incubated at 30°C for two weeks. Subsequently, the obtained isolate was identified by amplification and partial sequencing of 16S rDNA.
DNA extraction: Extraction of genomic DNA was performed by alkaline lysis of fresh colonies according to Von Post et al. (2003). The obtained DNA was quantified by spectrophotometry at 260 nm using the NanoDropTM 2000 (TermoFisher).
PCR amplification and sequencing: 16S rDNA gene from isolate was PCR amplified using bacterial universal primers 27f(5'-AGAGTTTGATCCTGGCTCAG-3') and 1492r (5'-GGTTACCTTGTTACGACTT-3') (Weisburg et al., 1991) and the premixed solution GoTaq® Green Master Mix (Promega Corporation, USA). PCR conditions were: an initial denaturation (95 °C, 10 min), 35 cycles of 92 ° C, 1 min; 50 °C, 45 s and 72 °C, 1.5 min; followed by a final extension (72 °C, 5 min). PCR was performed in an MS mini thermocycler (Major Science, USA). The PCR reaction mixture (25 µL volume) contained 1X GoTaq®Green Master Mix, 0.4 µM of each primer and 50 ng of DNA. The amplification product was verified by 1 % agarose gel electrophoresis at 80 V for 45 min. Sequencing was performed by the Sanger method in the company Macrogen® (Republic of Korea, http://www.macrogen.com/en/main/index.php).
Sequences edition and identification: Quality analyses of the sequences were performed with FinchTV1.4.0 (Geospiza, Inc). The obtained sequence was compared with the Gen Bank database using the BLASTn (Basic Local Alignment Search Tool) program of the National Center for Biotechnology Information (NCBI) (Benson et al., 2015).
RESULTS AND DISCUSSION
In vitro propagation of INCAM 11 (R. irregularis)
The monoxenic culture of R. irregularis INCAM 11 was successfully established in transformed chicory roots. Ri T-DNA transformed chicory roots show greater AM intraradical colonization and sustain higher extraradical hyphal development than non-transformed roots (Danesh and Tufenkci, 2017; Lalaymia and Declerck, 2020).
After 3 days of culture, spore germination and hyphal growth was observed, with simultaneous growth of germ tube towards hairy the roots (Figure 1). The first interaction between fungus mycelium and roots occurred 5-7 days after inoculation.
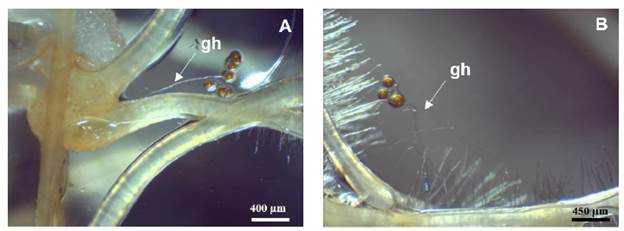
Figure 1 In vitro INCAM 11 (R. irregularis) spore germination. A (25X) and B (30X): Germinating hypha (gh) associated to Ri T-DNA transformed chicory roots after seven days of culture.
The germ tube produced multiply laterals branches on media surface and toward roots with extensive hyphal proliferation (Figure 2 B, C and D). Figure 2 (A) shows "fan-like structure" (FLS) formation after 5th day of cultivation. Hyphal length was recorded during 90 days.
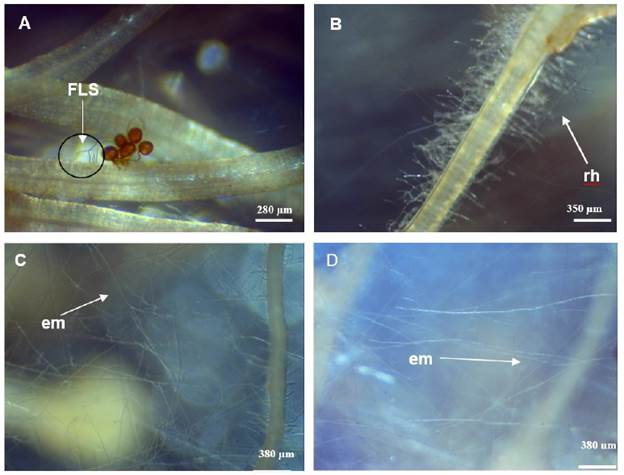
Figure 2 In vitro INCAM 11 (R. irregularis) hyphal growth. A: "Fan-like structure" formation (FLS) after five days of culture (50X). B: Runner hypha (rh) (30X), C and D: Extraradical mycelium (em) network C (30X), D (50X).
Some investigations under in vitro growth conditions revealed that, once the root-fungi contact was established, the fungal morphology changes drastically, with a reorientation of hyphal apical growth giving rise to either a direct entry point or to a hyphal branching called FLS, which may lead to the formation of an appressorium (Dalpé et al., 2005).
During the experiment, when hyphae reached the root surface the pattern starts to change, their growth became fast and in a straight way toward hairy roots. Hy-phae branched grew in all directions followed by hyphae proliferation intensively near the roots (Figure 2 B) and a rapidly increment of hyphal density into the medium (Figure 2 C, D). Danesh et al. (2016), reported in the in vitro culture of R. intraradices with carrot transformed hairy roots, the growth and dispersal of profuse extraradical mycelium in the culture medium. On the other hand, Costa et al. (2013), observed that in in vitro culture of Gigaspora decipiens and R. clarus produced typical structures like "branched absorbing structure" (BAS) after spore's germination. Even if the AMF species are different their behavior is similar in root organ culture conditions.
The mean hyphal length was increasing rapidly during the experiment dynamic reaching 865.6 cm length at week 13 (90 days) (Figure 3). This value is similar compared to those reported by other authors. Voets et al. (2005), reported from experiments conducted on potato plantlets with a R. intraradices strain a mean hyphal length of 1 300 cm at week 22, seven weeks more than in this experiment.
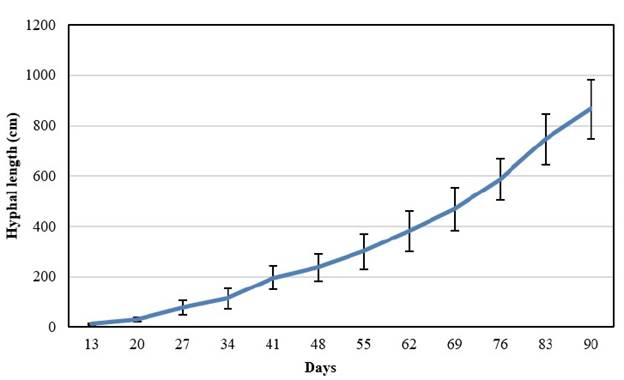
Figure 3 Hyphal growth dynamics of INCAM 11 (R. irregularis) associated with transformed chicory roots on MSR medium after 90 days of culture. Bars means confidence intervals, n=100.
Results from experiments developed by De La Providencia et al. (2005), on transformed carrot roots with two different strains of R. intraradices showed values of 1 831 cm and 1 442 cm in a period of 6 to 8 months. The architecture of the extraradical mycelium as well as the growth characteristics was similar to those observed in the classical monoxenic culture systems on excised root organs (Danesh and Tufenkci, 2017). Barceló et al. (2020), emphasized the importance of such extraradical mycelium networks to initiate rapid colonization in new hosts.
The formation of new spores began within 21- 25 days after association (Figure 4 A, B). The spore's formation rate was slow during first 60 days and increased progressively after 90 days of incubation (Figure 4 C, D). The spore number was around 2 000 per petri dish after 5 months of incubation. This result is in agreement with Voets et al. (2005), who observed the first newly produced spores at week 3.
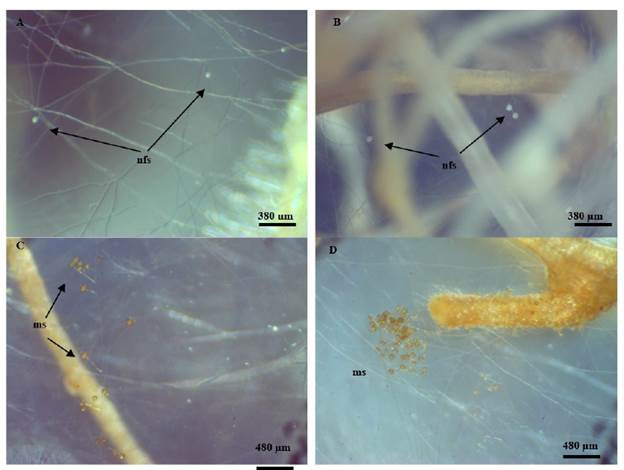
Figure 4 In vitro INCAM 11 (R. irregularis) spore's production. A, B: Newly formed spores (nfs) on extraradical mycelium (30X) after 21 days of culture. C, D: Mature spores (ms) formation on extraradical mycelia at 90 days of cultivation (30X).
First spores were smaller than mature spores and were formed almost terminally on short hyphal branches (Figure 4 A, B). These vegetative spores were hyaline whitish colored but turned to brownish yellow after several weeks and become in mature (Figure 4 C, D). After 5 months of culture matured spores with a light brown to a dark brown color were observed.
In this investigation, the rate of sporulation increased after 90 days of incubation. It might be due to the decrease of sucrose and other mineral component level in the medium (Srinivasan et al, 2014). Similar trend was observed by D'Souza et al. (2013), who showed that the rate of sporulation depended on the sucrose concentration of the media. Besides, the sporulation rate was not the same in all plates; such behavior it could be attributed to root - AMF communication in the pre-symbiotic and symbiotic phase, due it is a complex multi-step process under genetic control, regulated by an intimate molecular dialog between the host root and the obligate symbiont (MacLean et al., 2017). Such multi-step process could impact root colonization and subsequent spore production (Voets et al., 2005).
The spore's number produced by INCAM 11 is around 2000 per petri dish after 5 months of incubation, is low compared with Srinivasan et al. (2014), and Danesh et al. (2016). They recorded around 8 500 spores and 2 500 spores of R. intraradices after 3 months of culture, respectively. However, only a few AMF species are fast growers and colonizers that are able to produce many thousands in vitro propagules in a few months (Ijdo et al., 2011).
The newly produced spores were able to form associations with new hairy roots in vitro (Figure 5), following sub-cultivation, reproduced them self and completed their life cycle. Rhizophagus irregularis INCAM 11 in vitro spores have the ability to form new mycorrhizal associations after a subculture period indicating that this strain is capable to complete their life cycle under in vitro conditions. After 15 days of culture newly spore start to growth in the extraradical mycelium. They were small and transparent and after 95 days of incubation they exhibited general morphological similarity to soil borne inoculum (Figure 5 B), although they had thicker spore walls than their soil borne counterparts.
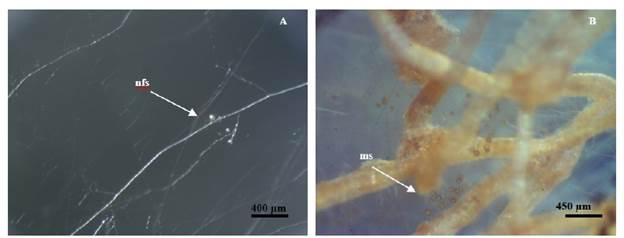
Figure 5 /n v/tro R. irregularis spore's production after sub-cultivation. A: Newly formed spores (nfs) after 25 days of culture (30X). B: Mature spores (ms) after 95 days of incubation (30X).
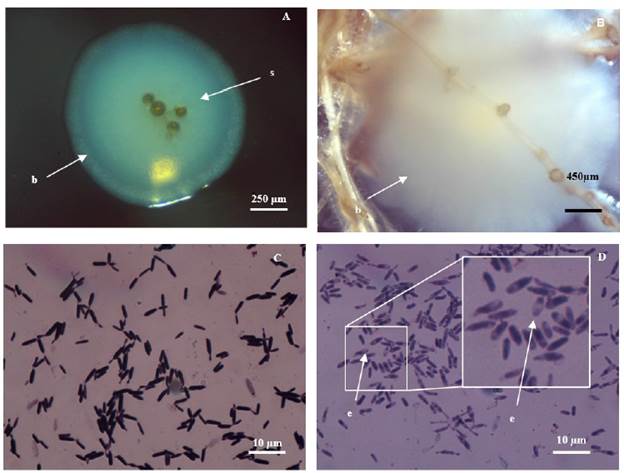
Figure 6 Bacterium isolated from INCAM 11 (R. irregularis) in vitro culture. A, B: spores (s) surrounded by an associated bacterium (b). A (Novel, 40X) and B (Novel, 8X). (C) Gram stain: gram-positive cells and rod shaped. (D) Bacterial endospore (e) (Novel, 1000X).
AM root-organ cultures facilitates the study of environmental, nutritional, and physiological effects on fungal growth and spore morphology. A range of parameters related to sporulation, hyphal architecture, and spore ontogenesis can now be compared according to the systematic approaches used (Danesh and Tufenkci, 2017). The multiple characteristics (anatomical, ultra-structural, or biochemical) that can be generated using in vitro produced AM fungal colonies need to be classified according to their taxonomic significance, to discriminate between environmentally related and phylogenetically driven taxonomic parameters (Kokkoris and Hart, 2019). For example, in vitro differentiated spores may be slightly smaller and are often less pigmented than soil-borne spores (Danesh et al., 2016).
Isolation and identification of the most frequent associated microorganism to the in vitro culture of INCAM 11
A microorganism colony of bacterial origin was found to be frequently associated with INCAM 11 spores (Figure 6 A and B).
Bacteria colonies were round shaped, white and medium size (2 - 4 mm), concentric, of convex surface, with rounded, flat borders and moderate mucosity (Figure 6 A and B).
After 30 h of incubation on Agar nutrient medium some microbial growth was detected. After application of the Gram stain, predominance of bacillary and Gram positive microorganisms (Figure 6 C) as well as bacterial endospores (Figure 6 D) could be observed.
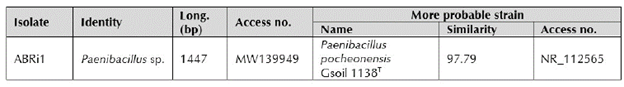
According to the 16S rDNA gene sequence, the strain ABRi1 was identified as Paenibacillus sp. (Table 1).
Table 1 Bacterial strain associated to INCAM 11 in vitro culture identified by partial 16S rDNA sequencing.
This bacterial genus commonly grows associated with in vitro culture of AMF strains. Bidondo et al. (2011), isolated Paenibacillus sp. associated with spores of Funneliformis mosseae. Accordingly, Hildebrandt et al. (2002), found that slime-forming bacteria, identified as P. validus, frequently pop up when spores of R. intraradices germinated on modified M medium concluding that these bacteria were able to support fungus growth on agar medium.
In the presence of P. validus, hyphae branched profusely and formed coiled structures. P. rhizosphaerae significantly increased the growth of extraradical hyphae and the number of newly formed spores of R. irregularis cultivated in vitro (Battini et al., 2017). Paenibacillus strains increased R. irregularis development and stimulates AM fungal root colonization, spore germination, and hyphal growth of F. mosseae (Turrini et al., 2018). Paenibacillus species are widely distributed in the mycorrhizosphere and could have a positive effect on the development of the in vivo and in vitro AM symbiosis (Bidondo et al., 2011).
Cruz and Ishii (2011) suggested that the most AM fungi-associated bacteria are located on the surface of spores and hyphae, although some bacteria have been found in the cytoplasm of AM fungi spores. They could stimulate spore germination by eroding spore walls, by producing stimulatory compounds such as CO2 and other volatiles, by influencing AM fungi phosphorus acquisition or by ability to hydrolyze biopolymers such as chitin and chitosan, the two main components of spore walls (Agnolucci et al., 2015; Turrini et al., 2018; Giovannini et al., 2020).
Roesti et al. (2005), showed that most of the bacteria found in association with spore walls of the AM fungi, F. geosporum and Septoglomus constrictum were able to degrade biopolymers such as cellulose and chitin. These authors suggested that the increase in germination of Glomus sensu lato spores could be related to the degradation of external layers of spore walls by soil bacteria. According to those authors some toxic compounds that inhibit germination of AM propagules and mycelial growth could be also reduced by these bacteria.
Bidondo et al. (2011), reported a significant promotion in pre-symbiotic mycelium development occurred after inoculation of two Paenibacillus species isolated from AM propagules under in vitro conditions. P. rhizosphaerae TGX5E significantly increased the extraradical mycelium network, the rate of sporulation, and root colonization on an in vitro symbiotic association. These results were also observed in the rhizosphere of soybean plants grown under greenhouse conditions, when P. rhizosphaerae was co-inoculated with R. intraradices. Species of Paenibacillus associated with AM fungus structures in the soil, may have a promoting effect on short term pre-symbiotic mycelium development, and little impact on AM propagule germination (Bidondo et al., 2011).
CONCLUSION
The in vitro system has proved to be a useful tool for the cultivation and conservation of a large number of species and isolates of AM fungi. The monoxenic cultures of Cuban strain R. irregularis INCAM 11 was successfully established on chicory transformed roots, providing spore production in a small space and over short period of time. Effects of Paenibacillus sp. on R. irregularis in vitro germination and establishment most be evaluated. Our results are the first reported in Cuba in terms of in vitro establishment of AM fungus and are the basis for the creation of an in vitro AM fungal collection.














