INTRODUCTION
Post-operative tissue repair and its underlying complications, such as infections or suture line dehiscence, are critical processes in patient recovery, which affect their welfare and productive performance. Epithelial healing and neovascularization of the subepithelial tissue are key processes in tissue repair. In fact, the epithelial barrier limits secondary infections and generate a hypoxic milieu allowing an efficient glucose uptake by the cells. This process increases cytokine production which help the scarring and angiogenesis (Hong et al. 2014; Sano et al. 2012)
Platelet-Rich Plasma (PRP) therapy improves and accelerates wound healing and regeneration, and it has been used in several species including human with promising results (De Pascale et al. 2015; Sommeling et al. 2013; Tate & Crane, 2010). However, its use in skin wounds requires in vivo studies to validate and support the findings described in vitro conditions. The present study assessed autologous PRP therapy in the epithelial regeneration of surgical-induced skin wounds in rabbits (Oryctolagus cuniculus).
MATERIAL AND METHODS
This study was conducted with the approval of the Bioethics committee of the University of Tolima (Act No 01 May 2016), and complied the guidelines of the Code of Ethics for the Professional Practice of Veterinary Medicine and Zootechny (Law 576 of 2000).
Eighth New Zealand male rabbits clinically healthy with an average weight of 3 ± 0.2kg were used. During the pre- experimental and the experimental period, the animals were kept at room temperature, provided with commercial feed (17% protein, Solla, Colombia), and water ad libitum. General anesthesia was provided using a combination of Xylazine (2mg/kg, Xylazine 2%, Erma®, Colombia) and Ketamine (6mg/kg, Ketamine 50, Holliday®, Argentina), intravenously. The jugular vein was externalized by dissection following protocol proposed by González et al. (2013). Local lidocaine infiltration (Lidocaine 2%, Virbac, Colombia) was performed in the subcutaneous tissue, close to the jugular vein. Six mL of blood were collected using two vacuum tubes (21G catheter) containing 3,2% sodium citrate (Vacuette®), and analyzed by means of automatized hemogram (FC 620VET, Fuerte Care, Colombia) to determine initial hematological values. Consecutively, the samples were centrifuged at 120g for 5min (Hermle® Z 32 HK centrifuge, Germany), and 1mL of the layer superior to the buffy coat of each tube was collected and analyzed by automated hemogram. Platelet collection efficiency (PCE) was determined using the formula described by Weibrich et al. (2003):
For the evaluation of the effect of PRP on skin wound healing, after to a skin shaved and antisepsis protocol, using a scalpel a clean epidermal surgical incision of 5cm length in cranial to caudal direction was made in the interscapular region and closed in simple pattern using surgical nylon suture. The rabbits were randomly distributed in two groups; group 1 (n=4) was treated with surgical nylon suture alone, and group 2 (n=4) received surgical nylon plus PRP therapy. Previous to the surgery, animals received prophylactic antibiotic therapy (Cephalexin 25mg/kg i.m, Rilexine® 150, Virbac, Colombia) and local analgesia was induced by epidermal nerve blocking from the interscapular region through subcutaneous lidocaine injections (Lidocaine 2%, Virbac, Colombia). Finally, PRP was activated by the addition of calcium gluconate (VECOL®, Colombia) in a 10:1 ratio and applied immediately over the wound after the suture was finished.
Cutaneous tissue biopsies were taken using a disposable punch 8mm in diameter (Kai®, Japan), on days 3, 7, 14 and 21 after surgery. The samples were stained with Hematoxylin-Eosin and with Masson›s Trichrome, and the cellular inflammatory response was evaluated by microscopy (counting of cell lines: neutrophils, macrophages and lymphocytes; and collagen fibers arrangement). Photomicrographs were obtained by digital camera (Olympus XC50) adapted to an inverted microscope (Olympus IX73) and visualized using cellSens Standard v1.12 (Olympus®, Japan). The minimum number of fields to count was determined following the guideline described in Moro et al. (2004).
Data were expressed as mean ± SE. Statistical significance was determined for PRP concentration using the Wilcoxon test. For cell line count of the histological sections, Mann Whitney test and post hoc Dunn multiple comparison test was used. Statistical analyses were performed with GraphPad Prism v 6.0 (La Jolla, CA, USA). P<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
The number of cells in the whole blood sample and PRP were compared. Significantly difference (p <0.05) was observed in the red blood cells count (RBC) and platelet number (PLT). PRP showed lower RBC and higher PLT than whole blood (Table 1). White blood cell count (WBC) were lower in the PRP, with p values close to significance (p = 0.0571). Mean platelet volume (MPV) and platelet distribution volume (PDV) did not show statistical difference. The PCE was 53.5% and the percentage of PLT in the PRP was 1.56 times higher (156%) than the basal level.
Table 1 Hematologic values in whole blood and PRP obtained from rabbits.
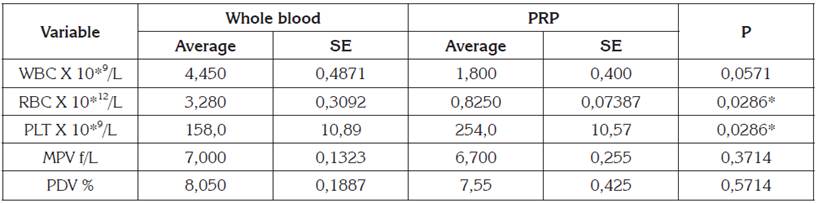
SE: standard error of the mean. P: p value in Mann-Whitney U test, *: statistically significant difference.
Previous studies have been showed the potential use of PRP in wound healing (Barrionuevo et al. 2015; Ding et al. 2017; Wang et al. 2016). Nevertheless, the quality of PRP and its final concentration depend on the protocol used. González et al. (2013) and Nagata et al. (2010) described that double centrifugation increases the platelet density obtained, but it is more sensitive to errors in the process and provokes early platelet activation, which decreases underlying the effect of growth factors on the lesion. Nonetheless, the integrity of the PRP obtained in the present study was preserved, evidenced in unaltered MPV and PDV values between PRP and whole blood.
By means of a unique centrifugation was obtained 156% platelet enrichment with reduced number of other cell lines, higher than reported in this species 128.5% and 80.73% (González et al. 2016; Nagata et al. 2010). Furthermore, PCE (53,5%) was higher than the reported for rabbits (18-21%) (González et al. 2013), canine (29,9%), equine (7,5%) and bovine (19,1%) (Argüelles et al. 2006; López et al. 2012; Silva et al. 2011), but similar to PCE values reported in felines (50%) (Silva et al. 2011). On other hand, the PLT number was lower than the reported by Nagata et al. (2010) and Pazzini et al. (2016) using double-centrifugation method. However, it has been observed that clinical response improvement has correlation with platelet integrity and not with the platelet concentration (López et al. 2012).
In the PRP preparation, anticoagulants such as heparin, EDTA, sodium citrate, citric acid, and dextrose have been used to preserved the blood sample (White, 2000). Sodium citrate was used due to the action to preserve the structure and homogeneity of platelets and is available in sterile vacutainer tube, reducing the risk of sample contamination. Additionally, sodium citrate did not induce changes on growth factors concentration in rabbit PRP (González et al. 2016).
The results of WBC in PRP was lower than obtained by González et al. (2013). Some authors consider that high concentration of WBC in PRP could be detrimental in the healing process (Chizzolini et al. 2011; Zimmermann et al. 2003) due to the accumulation of leukocyte infiltrate at the site of injury is key in initiating an exaggerated inflammatory response. Thus, a rabbit model has been generated to reduce WBC in the PRP separation with favorable results (González et al. 2016). The collect of blood by jugular vein dissection reduced the risk of vascular traumatisms (Pazzini et al. 2016) reducing the inflammatory response variability.
Histological analysis showed no significant changes between groups. At day 3, disorganized pattern of small collagen fibers proliferation and fibroblasts was observed. At day 7, hair follicles proliferation was found in the injured area associated to a best collagen fibers arrangement. At day 14, the epithelialization was complete with increased thickness of keratinized stratum. Remarkably, PRP treated group showed denser and uniform collagen fibers arrangement. At day 21, there was not distinction between injured and adjacent healthy tissue, indicating complete tissue repair (Figures 1 and 2).
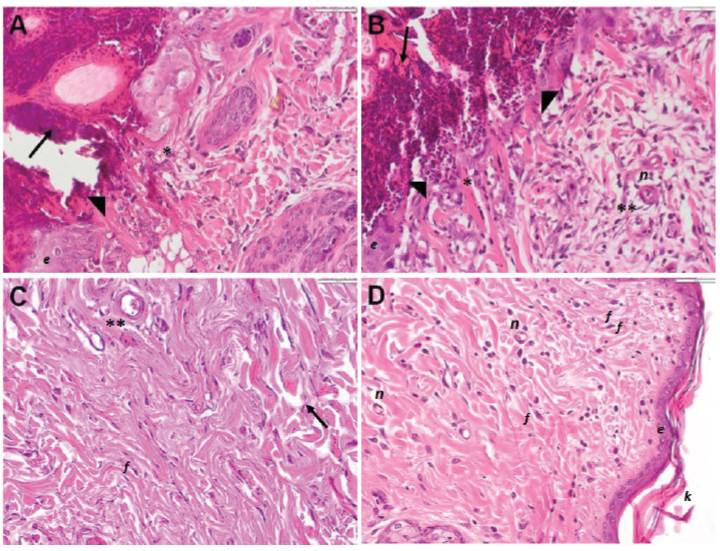
Figure 1 Histological features of skin repair in rabbits treated with PRP. Representative histological skin sections stained with H&E from group 1 (A, C) and group 2 (B, D). At 3-day post-surgery (A, B) a formation of clot in the reticular area (arrow), neutrophil infiltration is perceived (arrowhead), mainly in the sub-epidermal region (*) and adjacent to blood vessels (**) are observed. At 21-day post-surgery, (C) the control group showed dense and irregular connective tissue (arrow), with moderate cellularity in dermis. Similarly, (D) PRP group showed collagen fibers are observed in parallel, moderately organized in bundles and abundant cellularity. n: neovascularization f: fibroblast; K: keratin; e: epithelium. 40X magnification.
Lee et al. (2008) found a high number of tissue-infiltrating neutrophils in the initial healing stage. Similarly, Pereira et al. (2012) demonstrated which neutrophils infiltration is predominant in initial inflammation response against toxins in skin of rabbits. Besides, tissue-infiltrate macrophages observed, it is known play a role in the acceleration of tissue healing, mainly the M2 phenotype (Lemo et al. 2010; Seo & Jung, 2016). Although lymphocytes are not directly involved in initiating wound healing, these are crucial in decreasing susceptibility to septic process (Park & Barbul, 2004). The leuko-reduced PRP obtained is ideal for avoid an exaggerated immune response with the underlying consequences in the wound healing process (Dohan et al. 2009).
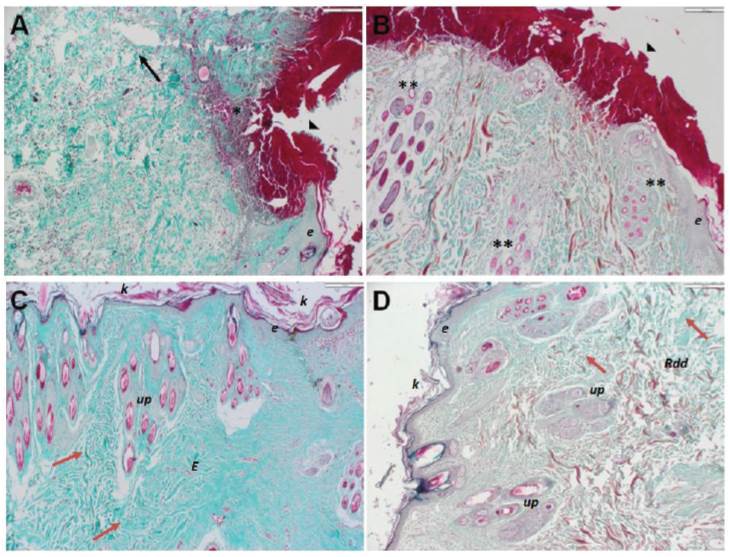
Figure 2 Collagen fibers in skin repair in rabbits treated with PRP. Representative histological skin sections stained with Masson’s Trichrome from group 1 (A, C) and group 2 (B, D) At 3-day post-surgery (A, B) characteristic clot formation is observed (arrowhead). Group 1 present separation of collagen fibers, edema (black arrow) and severe inflammatory cell infiltration (*), equally, group 2 shown an increased number of hair follicles (**). At 21-day post-surgery (C, D) irregular and dense connective tissue (red arrow), composed mainly of type I collagen fibers intensely colored in green are observed. Connective tissue sclerosis structuring the dermis (E). Rdd: reticular deep dermis; Up: pilosebaceous units; K: keratin; e: epithelium. 10X magnification.
Inflammatory cell density in the tissue did not show significant differences (Table 2) between group 1 and 2. Time-dependent cellular number reduction as the wound-healing process progressed was evidenced. Additionally, fibers length was higher in group 2 than group 1 (p <0.05), at 21th day (Figure 3).
Table 2 Inflammatory cell count in skin biopsies from rabbits.
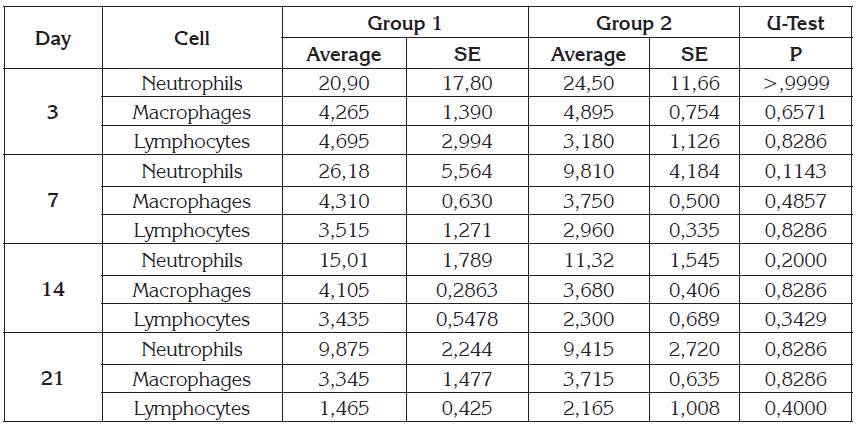
Mann Whitney U-test, SE: Standard error. Significance with p <0.05.
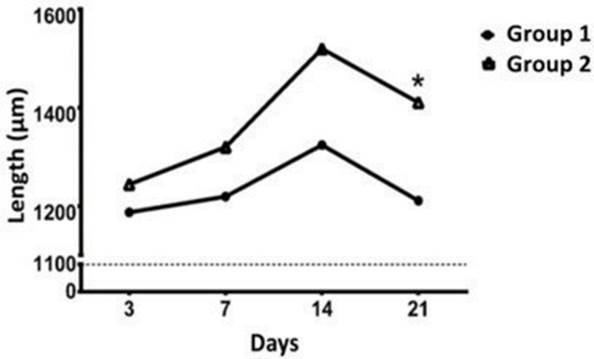
Figure 3 Collagen fibers length in skin section of rabbits treated with PRP at different time points. Collagen fibers length was measured at 3, 7, 14 and 21 days after surgery. *: p <0.05.
Sánchez et al. (2007) showed that the magnitude of inflammatory response to an injury varies depending on many factors, including the nature of the incisional instrument used to induce the skin wound, where scalpel incisions like the used, result in less inflammation. The PRP used elicited an inflammatory response similar to the control group. This could be beneficial considering that exuberant immune response is detrimental on tissue repair by promoting keloid and hypertrophic scar (Seo & Jung, 2016). Microscopic analysis showed homogenous collagen fibers proliferation in both treated and control skin. Leuko-reduced PRP as obtained increases the expression of genes for production of type I collagen fibers, which are favorable to restoring the normal composition of the injured tissue (González et al. 2016).
Briefly, this results showed that under the described condition it is possible to get a PRP enrichment with 156% more platelets than the basal levels and reduced number of erythrocytes and leukocytes. On the other hand, using autologous PRP did not generate an inflammatory response greater than the control group. However, the histopathological features revealed changes that could contribute to tissue repair. In conclusion, PRP could be useful for the management of this type of wounds collaborate in the epidermal tissue repair.















