INTRODUCTION
Quinoa, Chenopodium quinoa, is native to the Andes, and is distributed from southern Chile and Argentina to Colombia (Bazile et al. 2014), between sea level and 4.000m altitude (Veloza et al. 2016). It was introduced in North America, Africa, Asia and Europe (Bazile et al. 2014; Morillo et al. 2017). Cultivated in a traditional way since the Inca period (Delgado et al. 2009), standing out as a staple food in the diet of some peoples of South America (Torres et al. 2000). In Colombia, it is grown in the departments of Boyacá, Cauca, Cundinamarca and Nariño (Dueñas, 2014). In Boyacá, crops between 2.538 and 3.031m of altitude are reported in the provinces of Centro, Tundama and Sugamuxi (Veloza et al. 2016), with an average production of 5.37 tons and an average yield of 1.96 tons per hectare (Ministerio de Agricultura y Desarrrollo Rural, 2005; Departamento Administrativo de Planeación de Boyacá, 2016).
It is an expanding crop, due to the nutritional value of its fruit, high protein content, since it has all the amino acids, trace elements and significant amounts of vitamins B, C and E, as well as important minerals Ca, K, Fe, Mg, Mn, P and isoflavones that can contribute to their antioxidant properties (Montoya et al. 2005; Delgado et al. 2009; Vega et al. 2010; González et al. 2011; González et al. 2012; Prado et al. 2014; González et al. 2015; Morillo et al. 2017; Sayago et al. 2017; Reguera et al. 2018). Also, due to its capacity to adapt to different agroecological zones and its resistance to adverse abiotic factors, frosts, low levels of organic matter, salinity and drought (Veloza et al. 2016). Conditions that occur in the provinces of Boyacá where it is grown. In the last decade it has had an important recognition by governmental organizations at a national and international level, promoting actions aimed at replanting it and shaping its productive chain (Betancourth et al. 2007; Dueñas, 2014).
It is a small-scale crop in our country, where the indiscriminate mix of varieties, together with a low level of technology, reduce its quality and profitability (Veloza et al. 2016). Due to the lack of plantations of a single variety, technological problems arise, such as the heterogeneity in the morphological characteristics and the maturation times of the individuals (Delatorre et al. 2013; Dueñas, 2014).
There is information on the morpho-agronomic characterization of the Ch quinoa varieties cultivated in the savannah of Bogotá and in Nariño, where the height of the individuals, length of the inflorescence, seed weight, among others were evaluated (Torres et al. 2000; Delgado et al. 2009; Veloza et al. 2016); however, in Boyacá there has not been a study where the morphological characteristics of the cultivated varieties are described. It is necessary to carry out studies that allow a correct selection of the material, with a view to improving the production process. Therefore, the objective was to recognize the reproductive characteristics, vegetative and some physiological parameters in three stages of development, seed, seedlings and adult plants, which allow identifying the six varieties, Amarilla Maranganí (AM), Blanca de Jericó (BJ), Blanca Real (BR), Negra (NE), Piartal (PI) and Tunkahuán (TH); known by the peasants of the region and who have not been given a morphological characterization.
MATERIALS AND METHODS
The characterization was carried out in three stages of the biological cycle of Ch. quinoa in the Laboratory of Genetics and Molecular Biology of the Pedagogical and Technological University of Tunja.
Seeds: The varieties, AM, BJ, BR, NE, PI, and TH, obtained from the Germplasm Laboratory of the Gobernación de Boyacá-Colombia, were analyzed, to which the following measurements were taken: Diameter: to 10 seeds by varying the diameter was measured with a micrometric screw of 0.0- 25mm. Weight: a group of a thousand seeds per variety was registered weight, with the scale Lexus, Model Mix-A. Germination tests: 50 seeds were taken per variety and placed separately in Petri dishes on filter paper moistened with distilled water. After 24 hours, we counted the number of seeds that showed rupture of the testa and the emergence of the radicle.
Seedlings: Follow-up to the development of seedlings: 48 seeds of the six varieties were planted, in germination trays and with peat as a substrate, divided into three replicas. This test was carried out 4 to 5 days after the emergence of the substrate, in Tunja, at an altitude of 2810m. A total and long height of the lamina of the cotyledons: measurements made on days 5, 6, 14, 17, 28, 35 and 49 of the development. The height was measured from the substrate to the apex of the seedling. Reddish pigmentation: Product of betacyanins (González et al. 2012; Masaaki, 2014; González et al. 2015; Sayago et al. 2017), was evaluated qualitatively according to the weak, medium and strong states, determined by direct observation.
Adult plants: Four varieties were considered, AM, BJ, PI, and TH, of crops established in the following locations, BJ and TH in the municipality of Siachoque-Province Center, altitude 2.760m; AM in Tunja-Province Center, 2.810m and PI in Tibasosa-Province Sugamuxi, 2.538m. Ten healthy plants were selected, free of diseases, pests and nutritional deficiencies. The following data were recorded: height of the plants; root: length of the primary and secondary roots; stem: color, diameter, presence of ribs and pigmented axils; leaves: color and shape of the petiole; width, length and number of teeth of the blade; panicle: shape and color (Font-Quer, 1979; Moreno, 1984).
Finally, the extended description of the species was made, based on the reproductive and vegetative characteristics of the varieties studied. Two comparative tables between the varieties were proposed and a taxonomic key, which included characters of the adult plants (Tables 3, 4).
Experimental design: For the size of the seeds, a completely randomized, balanced design was used, at 10 seeds per variety, with the variety being the main factor, and diameter the response variable. To monitor the development of seedlings, we worked with 48 seeds of the six varieties, in a factorial design, with blocking factor days of measurement. The main factor consisted of the variety and the answer variables were the height of the seedling and the length of the lamina of the cotyledons. The vegetative characteristics were analyzed with a completely randomized design, with a factor corresponding to the variety. The response variables, length of the petioles, length and width of the plates and the number of teeth of the nomophiles were studied independently.
The quantitative variables were analyzed by different statistical tests according to their relevance: Analysis of Variance, Multiple Comparisons of Tukey, Significant Minimum Difference-DMS and Bonferroni, considering significant statistical differences of less than 5%. In a complementary way, Pearson correlation tests were performed for the quantitative variables of the nomophiles. Additionally, the technique of Principal Component Analysis was applied, which allows to synthesize information and reduce the dimensionality of a data set (Zar, 1996; Pérez, 2004). The statistical analysis was carried out with the R programs, version 3.4.3 of free distribution and Spss, version 21.
Through the multivariate technique of cluster analysis, we sought to identify groups of quinoa varieties, according to their level of similarity in four variables: leaf length and width, length of petiole and number of teeth. Its graphics representation through the dendrogram facilitates the interpretation of Cluster analysis results, which, when used in a complementary manner with those obtained in the ACP, can complement results. The dendrogram was built with the software Minitab version 16, main menu statistics, with the multivariate instruction and conglomerate of variables, applying the Ward hierarchical grouping method and the Euclidean distance.
RESULTS AND DISCUSSION
Seed: The rapid appearance of the radicle after a few hours of imbibition (12 hours) agrees with the observations of Pitzschke (2018). FAO (2014) recommends that the germination percentage of stored seeds must exceed 85% so that these are profitable. Seeds of NE and PI did not exceed 70% germination (Table 1). The low germinative power of these two varieties could be related to a long period of storage of the seeds, and therefore a loss of latency, genetic erosion and low production (Alanoca et al. 2013; López & Maldonado, 2013). While AM, BJ, BR, and TH their germination percentage exceeded the 85% recommended by FAO (2014).
Table 1 Characteristics of the seeds.
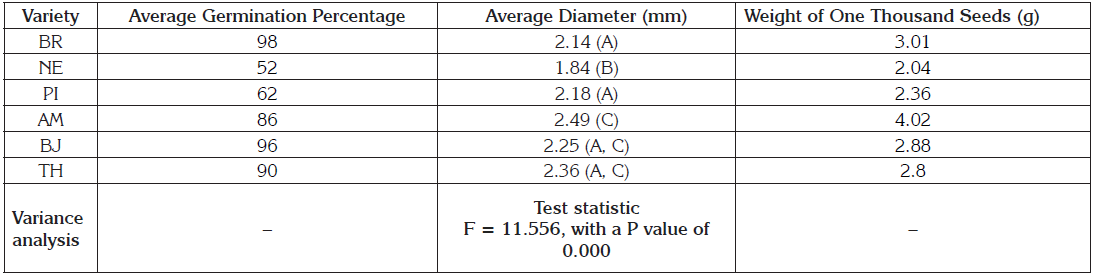
ANOVA: Bonferroni, DMS and Tukey. Stocks with a common letter -between parentheses- are not significantly different (p> 0.05).
The values of the analysis of variance and the multiple comparison tests of Bonferroni, DMS, and Tukey indicate that there are significant differences with respect to the diameter of the seed, where the variety NE presents the lowest average, 1.84mm and differs from the other five varieties. The varieties AM 2.49mm, TH 2.36mm, and BJ 2.25mm have the highest values and there are no significant differences between them, so they would form a group C, under a level of significance less than or equal to 5% (Table1).
Vilche et al. (2003) proposed three ranges to classify Ch. quinoa seeds according to their diameter: large 2.2-2.6mm; medium 1.8-2.1mm and small 1.0-1.7mm. In the same way, the seeds of AM, BJ, and TH are large while those of BR, NE and PI are medium (Table 1). Considering the weight of a thousand seeds and as reported by Delgado et al. (2009), could be taken as varieties of large grain AM and BR, since the weight of a thousand grains exceeds 3 grams. Likewise, the BJ and TH varieties have a medium grain since the weight of a thousand grains was between 2.5 and 3.0 grams. Finally, the NE and PI varieties, when having weights less than 2.5 grams, could be considered small grain varieties. The characterization of the seeds showed that AM possesses the grain with greater diameter and weight between the compared varieties, while NE and PI have the lowest values. From the commercial point of view, the size of the grain is of great importance (Mujica et al. 2004), AM, BJ and TH varieties are of greater interest for Boyacá producers, since according to their size and weight they can be considered as varieties of the special class (IBNORCA, 2002).
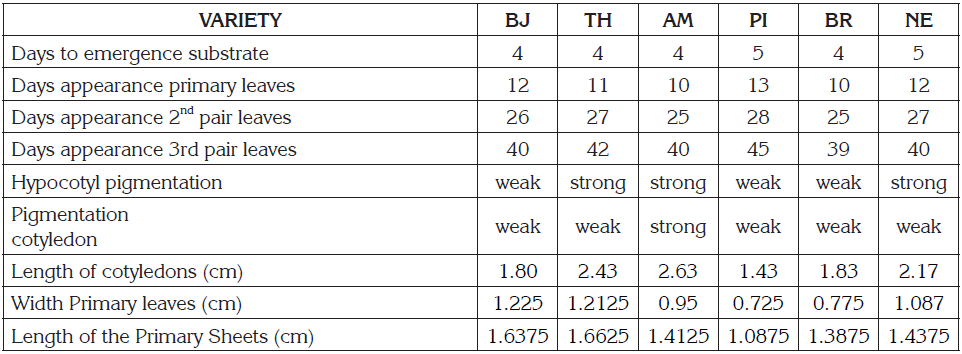
Seedlings the NE and PI varieties emerged on the fifth day, taking one day longer than the other varieties: Betancourth et al. (2007) report up to six and seven days for the emergence in the BJ and TH varieties, in tests done in the department of Nariño at altitudes of 2.500 and 2700m. In general, the NE and PI varieties require a greater number of days until reaching the phenological stages evaluated, while the varieties AM, and BR are the earliest (Table 2). According to the analysis of variance, significant differences were found in the varieties in terms of the seedling height (F = 1339,8; p = 0,000) and the length of the sheets of the cotyledons (F = 174,3; p = 0,000). The results of the multiple comparison tests of Bonferroni, DMS, and Tukey showed that NE and PI are grouped because they have a lower growth than the other varieties during all the days of measurement (Figure 1, Panel I). Likewise, AM presents slightly higher growth. With respect to the length of the cotyledons, AM also stands out for the higher average, while PI showed the smaller cotyledons (Table 2). Results that coincide with Boubaker et al. (1999), who report that large seeds produce seedlings with higher growth and the length of cotyledons. BJ and TH showed the highest average width and length of the primary leaves, while BR and PI presented the smallest leaves (Table 2). The Pearson correlation test showed a high relation (0,9) between the width and the length of the leaves. All varieties showed pigmentation in the hypocotyl and cotyledons coinciding with that reported by Torres et al. (2000), for 18 varieties. The pigmentation increases on the seventh day, after sowing, according to what was reported by Paśko et al. (2009). Pigmentation of the hypocotyl allows to differentiate the varieties into two groups, BJ, BR, and PI with weak coloration and in AM, NE and TH it is strong. In addition, the strong coloration of the cotyledons in AM the difference of the other varieties (Table 2).
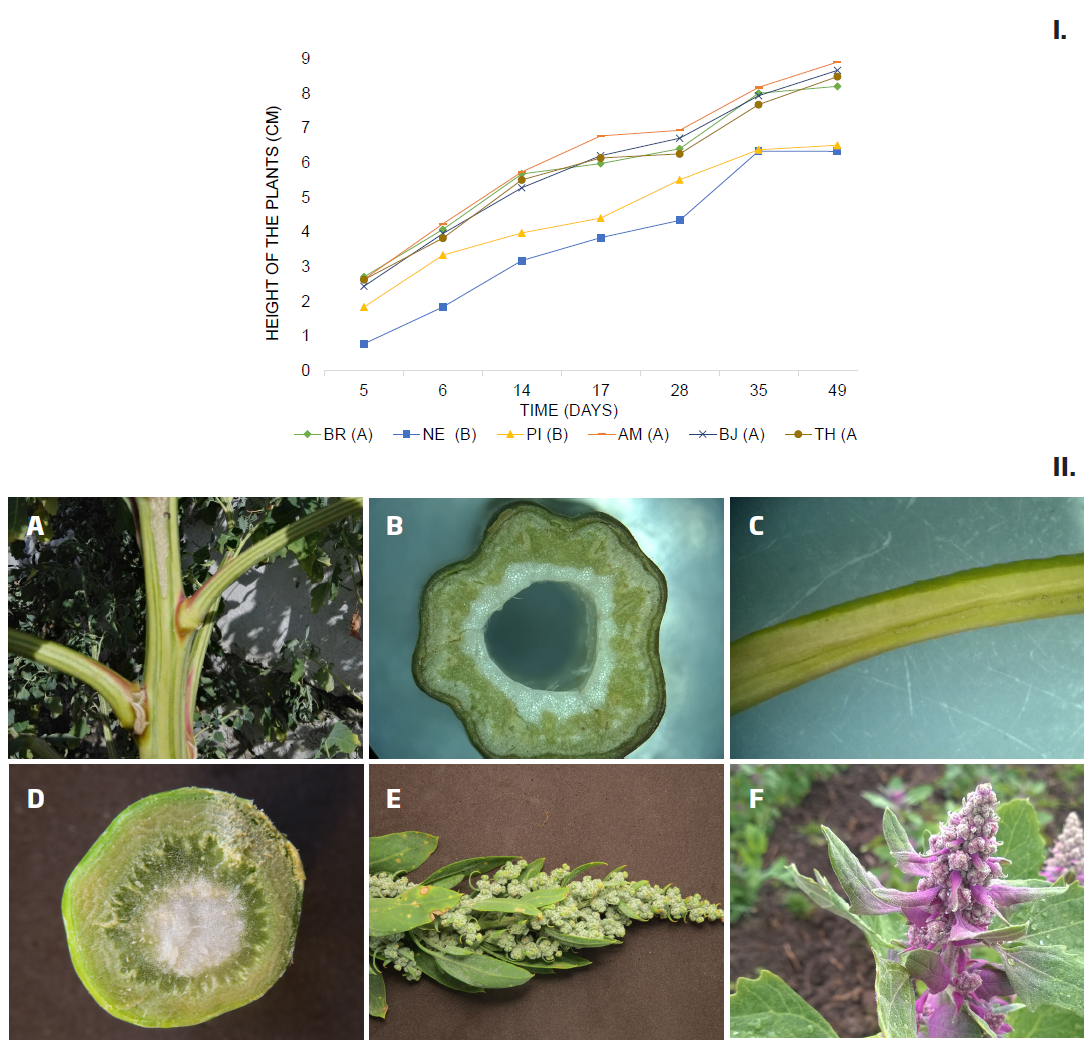
Figure 1 Characteristic of Ch. quinoa. Panel I. Average height (cm) of the seedlings according to variety and days of measurement. ANOVA Test: Bonferroni, DMS and Tukey. Varieties with a common letter -between parentheses- are not significantly different (p> 0.05). Panel II. Morphological characteristics in Ch. quinoa varieties: A) Discolored stalk and axils with red or purple epidermis in PI; B) Cross section of stem with ribs in AM; C) Petioles channelized adaxially in AM; D) Stem without ribs in BJ; E) Inflorescence green-dark and green-glaucous BJ; F) Purple inflorescence TH.
Table 2 Qualitative and quantitative data obtained from the monitoring of the development of the seedlings until the appearance of the third pair of leaves.
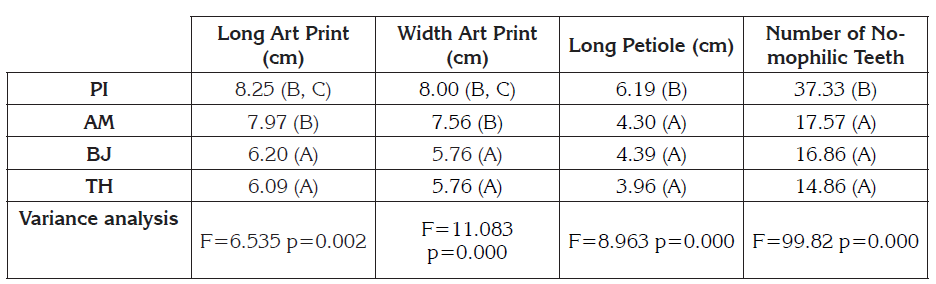
Adult plants: The PI variety presents the highest values in the width and length of the sheet, the number of teeth in the margin and length of the petiole (Table 3), however, it does not exceed the maximum values described by Torres et al. (2000) for these same variables. The analysis of variance showed significant statistical differences between some varieties, with respect to the width and average length of the sheet (Table 3). The tests of multiple comparisons showed that between the BJ and TH varieties there are no significant differences with respect to the mean width and length of the sheet, nor between the AM and PI varieties. There are significant differences in the average length of the petiole. The multiple comparison tests showed that the PI variety with a longer length is the only one that differs from the others (Table 3). There were statistically significant differences in the average number of teeth of the nomophiles. The Bonferroni, DMS and Tukey tests again concluded that the PI variety is the only one that differs from the others with a higher value in the average number of teeth of the nomophiles. According to the Pearson test there is a significantly high correlation (0,847) between the width and length of the lamina, as well as a medium-high correlation (0,672) between the length of the petiole and the number of teeth of the nomophiles, which coincides with the described by Torres et al. (2000), who found a positive-high correlation between these. The correlation between the length of the long-leaf petiole (0,442); length of the lamina-number of teeth of the nomophiles (0,463); width of the blade-length of the petiole (0,475) and width of the blade-number of teeth of the nomophiles (0,536) is medium and are significant at 5%. Given that Ch. quinoa has a very wide geographical distribution, both altitudinal and latitudinal, it is possible that leaf morphological structures vary depending on the environment and their genetic plasticity (González et al. 2014). According to Cabrol et al. (2014), intense UVB radiation is common in the Andes, due to the high altitude and the thin atmosphere. The decrease of the foliar surface can be related to a better adaptation to environments with high irradiation (González et al. 2014; Ivanova, 2014), as the environmental conditions present in the department of Boyacá, however, only physiological adaptations have been confirmed in quinoa at increasing doses of UVB radiation (Reyes et al. 2018).
Table 3 Quantitative data of nomophiles.
ANOVA: Bonferroni, DMS Tukey. Stockings with a common letter are not significantly different (p> 0.05).
Figure 2, Panel I, shows that the variables, length of the petiole and number of teeth have the greater association with the PI variety. The long and wide variables of the lamina have a relative association in AM. With the first two components, 88,1% of the variability is explained. The eigenvectors allow obtaining the coefficients with which each original variable was weighted, to confirm the main components CP1 and CP2. The variable width of the sheet receives the highest positive weight (0,532), all other variables receive positive weights on axis 1 (CP1). It stands out on axis 2 (CP2), the long variable petiole receives the highest positive weight (0,560), and the length of the blade receives the highest negative weight (-0,517). These variables are what most differentiates the varieties in this axis.
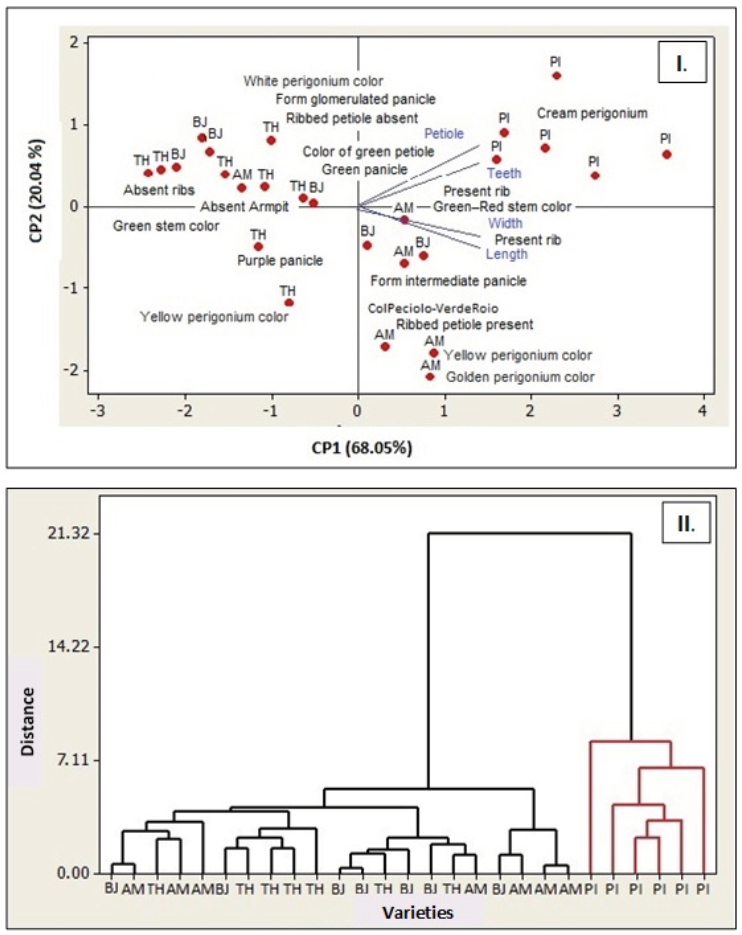
Figure 2 Qualitative characteristics and Euclidean distances in varieties of Ch. quinoa. Panel I Biplot graph, main components of the quantitative variables and their relationship with Ch. quinoa varieties. Panel II. Dendrogram from Euclidean distance for four quinoa varieties, based on four quantitative variables.
Aggregation of quinoa varieties according to quantitative variables (blade length and width, petiole length and number of teeth), Ward’s hierarchical clustering method and Euclidean distance allow the identification of two groups with a similarity of 77, 36 (Figure 2, Panel II). The group (1) of red color consists mainly of the varieties BJ, TH, and AM, the group (2) of blue color, formed only by the variable PI. Thus, in the dendrogram the confirmation of the clusters or conglomerates is reflected, as well as the distance among them, reflecting the greater distance of the PI variety, and greater closeness between the BJ, TH and AM varieties, in terms of the variables studied.
Enlarged Description. Enlarged description of Chenopodium quinoa: Based on the reproductive and vegetative characteristics of the varieties studied (Table 4).
Table 4 Comparative table of morphological characteristics in the four varieties of Chenopodium quinoa studied.
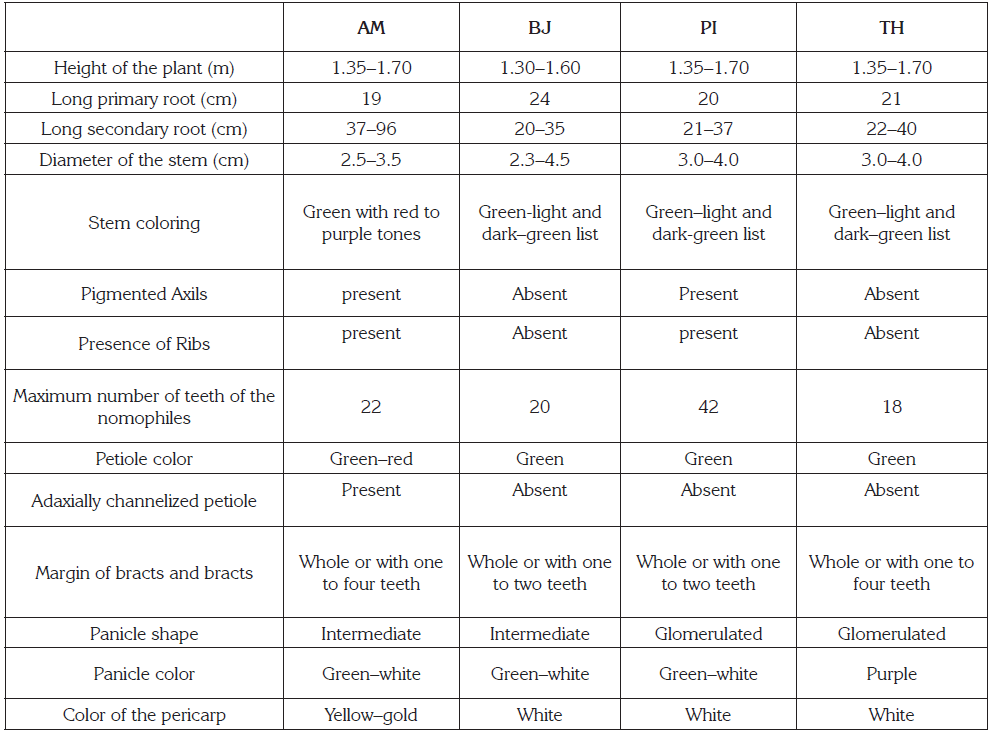
Plant polygamous-monoecious, herbaceous, annual, erect, 1,30-1,70 m high, with ascending branches. Fibrous root, axonomorphic, woody, root system allorrizia, primary root up to 25cm and secondary roots up to 96cm long. Cylindrical stem, with six to eight ribs, radial symmetry, 2,3-4,5 cm in diameter, discolored, with or without pigmented axils or ribs up to 5 mm high, porous and easily compressible marrow. Leaves deciduous, alternate, simple, membranous, deltoid to trullate, margin erose, of up to 42 teeth, apex obtuse to mucronulate, oblique base; actinodromous, basal and reticulated venation; opaque, pustulated surface-tiny blisters of a fraction of a millimeter-covered by small glands and petiole green or green-red, adaxially channelized or without longitudinal groove. Bracts and lanceolate bracts, entire margin or with one to four teeth and completely covered with glands. Inflorescence racemose-paniculated, glomeruled, intermediate or amarantiform. Actinomorphic flower, hypogynous, complete and incomplete, hermaphrodite or unisexual, perfect or imperfect, homochlamydeuos; dialycepetalous, calycine, green with white margins, covered by papillae translucent to purples; five stamens let’s stand on curved filaments, antithesis; antigens biloculars, dithecas, versatile, connate, parallel and introrse. Apocarpous gynoecium, superior ovary, apical style, and bifurcated stigma. Fruit simple, dry, indehiscent, achene, monosperm, white or orange pericarp, in the mature state with remnants of perigonium. Albuminous seed, 1,8-2,5mm in diameter, peripheral embryo, vitreous episperm.
TAXONOMIC KEY
1. Stems with ribs and epidermis of the red or purple axils Figure 1, Panel II, A-B). Margin of the nomophiles with 20-40 teeth (Table 4) …...............…..............................2
Reddish and adaxially canalized petiole (Figure 1, Panel II, C) ……….......................................AM
2’. Petiole green and without the longitudinal groove.........PI
1’. Stems without ribs and with the epidermis of the axils of the same color as the stem (Figure1, PanelII,D). Nomophiles with less than 20 teeth………………..…................................3
3. Inflorescence green-dark and green-glaucous (Figure 1, Panel II, E) …................................................................BJ
3’. Purple inflorescence (Figure 1, Panel II, F)....................TH
According to the altitude at which the crops were found, 2.538-2.810m, these are adapted to the ecological conditions of the inter-Andean valleys (Jellen, 2014; Graf et al. 2016; Zhang et al. 2017).
Adult plants were found, constant morphological characters such as the presence of ribs, pigmented axils and number of teeth in the nomophiles, which allowed recognizing the cultivated varieties in the department of Boyacá, see the taxonomic key (Tables 3, 4).
The problem facing growers in Boyacá is the planting of a mixture of varieties, which produce different grain sizes and saponin concentrations, among other characteristics, which affects the quality and yield of the derived products and therefore their commercialization. These characterizations make it possible to document the varieties in written form, to broaden the ancestral knowledge of the farmers in Boyacá, which serve as an input to produce homogeneous crops and certified seeds of each variety, which would solve the problems.














