INTRODUCTION
Schizolobium parahyba (Vell.) S.F. Blake (tambor or guapuruvu) - Fabaceae (Leguminosae, Caesalpinioideae) is a forest species native to the American tropics and present in the department of Córdoba, Colombia. It is a fast growing tree and can reach a height of 40m, in its adult state. It is considered one of the 12 most important species for reforestation and is widely used in the recovery of degraded areas in Colombia (Athanázio-Heliodoro et al. 2018; Lima et al. 2000; Salgado et al. 1989). Its wood is not very resistant to transformation and is used in the construction of river boats in various communities near rivers in some countries of the American tropics, including the northwestern area of Colombia. Due to the ease of carving and shaping it; it serves in light constructions, cabinetry, packaging, agroforestry systems and in parks, gardens and squares of various cities as an ornamental for its colourful yellow flowers, as well as in landscape projects (Trujillo, 2013). Also, the quality of its wood has been demonstrated to produce paper and cellulose (Nisgoski et al. 2012).
The fruits are pods, and each pod has one large, and sometimes two, round, flattened, and hard seed that is 2 to 3 cm long and 1,5 to 2,0 cm wide. It has between 1.250 and 1.600 seeds per kilogram. Fresh seeds have percentages of 70 to 90% germination (Athanázio-Heliodoro et al. 2018; Trujillo, 2013; Ferreira et al. 2007). The storage of seeds in a cold room reduces their average germination percentage between 40 and 50% and take two to three weeks to complete germination; with pre-germination treatments they can germinate up to 90%, from the third day and it is completed in two weeks. Its storage is recommended in airtight aluminum containers, at a temperature of 4ºC and a moisture content of 4,9%, for up to three years (Trujillo, 2013).
The species S. parahyba is present in the Colombian northwest and in the municipality of Tierralta, department of Córdoba, Colombia, in natural populations. It has been prioritized in the Colombian Caribbean by small, medium and large reforesters, reforestation and wood processing companies and the Córdoba Forest Chain, as one of the potential native forest species for reforestation and future forest plantation development. However, basic aspects related to morphometry, viability, seed germination and seedling vigour are unknown, which is essential to define their multiplication, establishment and natural regeneration, given that there is a high rate of deforestation within its distribution areas with unsuccessful regeneration, due to the extraction of trees, with desirable commercial characteristics (Espitia et al. 2017 a; Moreno & Del Valle, 2015).
This species, due to the agro-ecological conditions of the department of Córdoba, has been prioritized for the regeneration of the natural forest and the establishment of plantations for wood production, due to its adaptation, wood quality and various uses (Espitia et al. 2017 a). However, the largest effort in forestry research has been directed at species selection, forestry, development and marketing of forest products (Rodríguez, 2000), with technological limitations in the knowledge of sexual and asexual reproduction systems and seed certification systems (Nieto, 2004).
Studies related to the morphological characteristics, dimensions and weight of seeds of native and exotic forest species have been carried out by several authors to determine the viability, germination, behavior and adaptation of species in different ecosystems, conservation systems, use in nurseries and forestry. Trees, such as Copaifera langsdorffii Desf. (Fabaceae) and Schizolobium parahyba (Vell.) S.F. Blake (Fabaceae) have been studied in Brazil (Fogaça et al. 2011; Ferreira et al. 2007). In Colombia, studies have been reported on seeds of Bombacopsis quinata (Jacq.) Dugand (Malvaceae), Anacardium excelsum (Bert. et Balb) Skeels (Anacardiaceae), Cedrela odorata L. (Meliaceae) and Cariniana pyriformis M. (Lecythidaceae) (Espitia et al. 2017 a); also in seeds of Alnus jorullensis (Betulaceae), Cariniana pyriformis, Cedrela odorata, Cordia alliodora (Boraginaceae), Tabebuia rosea (Bignoniaceae), Anacardium excelsum, and Cedrela montana (Meliaceae) (Rodríguez & Nieto, 1999; Rodríguez, 2000).
There are several methods to determine the germination capacity of seeds, the best known and most practical is the germination test (Rao et al. 2007), which requires 30 or more days, which is too extensive. The ISTA only accepts three rapid methods of assessing viability, as official: the embryo excision, the tetrazolium topographic test and the X-ray method (ISTA, 2014). The tetrazolium test stands out for its speed, since the results can be obtained in approximately 24 hours or earlier, depending on the species, and its reliability has been tested in the evaluation of the quality of seeds of forest species, such as Parkia multijuga (Costa et al. 2018), Libidibia ferrea (Costa-Carvalho et al. 2017), B. quinata and A. excelsum (Espitia et al. 2017 a), Poincianella pyramidalis (Macedo-Sousa et al. 2017) and Copaifera langsdorffii Desf. and Schizolobium parahyba (Vell.) S.F. Blake (Fogaça et al. 2011; Ferreira et al. 2007).
Due to the economic and ecological importance of this species for the American tropics, northwestern Colombia and the Colombian Caribbean, the objective of this study was to describe the morphometric characteristics and viability of S. parahyba seeds.
MATERIALS AND METHODS
Localization. The research was carried out in the Plant Breeding Laboratory of the University of Córdoba (Montería, Colombia), located in the middle area of the Sinú Valley, at 8° 52' north latitude and 76° 48' west longitude, at a height of 13m. a.s.l. The ecological zone corresponds to the tropical dry forest, with an average temperature of 28ºC, relative humidity of 84% and annual precipitation of 1200mm (Palencia et al. 2006).
Genetic material. Freshly harvested free-pollinated sexual seed was used from commercial plantations at three Tierralta sites (Córdoba, Colombia). The trees are located between 70 and 100m. a.s.l., in the tropical dry forest ecological zone, with an average temperature of 27 - 28ºC, relative humidity of 84 - 86% and annual precipitation of 1200 - 1400mm (Palencia et al. 2006). The plantations are located at the following coordinates: 1) LN: 08º04'22,04'' and LO: 076º10'38,62''; 2) LN: 08º02'00,69'' and LO: 076º11'52,29''; 3) LN: 08º08'50,19" and LO: 076º07'59,95".
Since the species is allogamous (Trujillo, 2013), the seed of each tree, was considered as a half-sibling family. At the time of seed collection, the trees had ages between 10 and 25 years, height between 12 and 26m and stem diameter at 1.3m from 18 to 30cm.
Morphometric characterization of the seeds. For the morphometric characterization of the seeds, in each plantation, five trees were randomly taken and from each tree five samples of 100 seeds, for a total of 500 seeds. The response variables were: maximum width (AS), maximum length (LS), width/length ratio (RALA), measured in cm; while the fresh weight of a seed (PES) and fresh weight of 100 seeds (P100S), were measured in g. The number of seeds per kilogram (NSKG) was estimated by counting the number of seeds in five samples of 100 seeds; then the average was taken to kg, by the respective expansion factor.
For the morphological description and determination of topological patterns in the laboratory, the essential parts of 10 complete and healthy seeds, taken at random, were described based on the methodologies proposed by Niembro (1988) and Flores (2010), for seeds of trees and shrubs. For the study of the seed internal structure, mechanical scarification was performed, with the help of a file, on the seed coat towards the distal end of the cotyledons, as recommended by ISTA (2014), for hard seeds of the Fabaceae family. Then, they were immersed in distilled water for 48 hours at room temperature (between 25ºC and 30ºC); subsequently, longitudinal cuts were made through the embryo and half of each seed was discarded (Niembro 1988; Flores 2010).
Viability test. To evaluate the topological patterns of seed viability, a sample of 10 seeds replicated three times was used, which was stained with 2, 3, 5 triphenyl tetrazolium chloride, by immersing seeds in 1% solution, with a staining time of 2 hours, in the absence of light and at a temperature of 40ºC (Espitia et al. 2017 b). The seeds were then washed three times with distilled water, to remove excess dye and feasibility was assessed, with the help of a stereoscope (Vista Visión®), to improve the visualization of internal structures (MAPAB, 2009).
The seed evaluation was carried out by identifying three categories, recommended by Rao et al. (2007), for the interpretation of staining patterns:
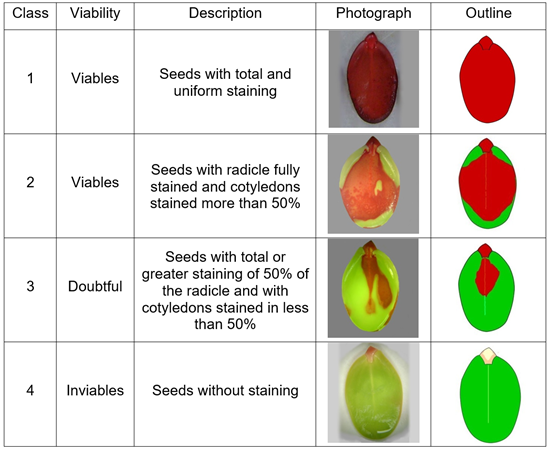
Category 1. a) Viable seeds: those with the embryonic axis and cotyledons completely stained/dyed; b) those that present superficial necrosis in the middle of the cotyledon, mainly in the parts far from the embryonic axis; c) those with unstained (dead) areas on the cotyledons, in places opposite the radicle.
Category 2. Non-viable seeds. a) Those with embryonic axis and unstained (dead) cotyledons; b) those with unstained embryonic axis, although the cotyledons are stained; c) those with acute necrosis in the embryonic axis; (d) those with slightly stained embryonic axis and unstained cotyledons; (e) those with necrosis at the tip of the radicle; f) those with serious damage to more than half of the essential parts of the seed.
Category 3. Doubtful seeds: Partially stained seeds will produce normal or abnormal seedlings, depending on the intensity and staining pattern. In this category are seeds that are less than half stained and with healthy essential parts.
To determine the optimal staining pattern, which defines seed viability, an experiment was established under a completely randomized design, with six treatments and four repetitions of 25 seeds each. The six treatments corresponded to the combination of three concentrations of tetrazolium (0.5, 1.0 and 1.5%) and two staining times (2 and 3 hours), structured as six levels of one factor. Viability was evaluated using the categories, described in the staining patterns, previously defined. The soaking, cutting and exposure of the embryos in each treatment was carried out in the same way, as previously described, in determining the topological patterns. Embryo staining efficiency was evaluated, based on intensity and color uniformity (Pinto et al. 2009). For this purpose, the types of stains that characterized each treatment in the seeds, which were photographically recorded, were analyzed with a digital camera. To consider the percentage of viable seeds in this test, the total seeds of the category "Viable" was taken, plus half of the seeds in the category "Doubtful" (Pinto et al. 2009).
The tetrazolium test was compared with a germination test under laboratory conditions to analyze the viability results with germination. A germination chamber (Dies®) was used, at a temperature of 28ºC, relative humidity of 80%, with a light period of 10 hours per day. For this purpose, four repetitions of 25 seeds each, arranged on paper towels, in aluminum trays were used and uniform irrigation was provided for 45 days (Espitia et al. 2017 b). Seed germination was evaluated by recording the number of normal and healthy seedlings emerged during the test.
Experimental technique and statistical analysis. For morphometric characteristics, descriptive statistics were performed and confidence intervals of 95% probability were estimated. To estimate the effects of tetrazolium treatments, an experiment was carried out under a completely randomized design, with six treatments and four replications of 25 seeds each. The six treatments corresponded to combinations of three tetrazolium concentrations (0.5, 1.0 and 1.5%) and two staining times (2 and 3 hours), structured as six levels of a factor. Analysis of variance was performed with six treatments and Duncan's multiple range test, at 5% probability. The free access computer program, Windows GENES version V.2014.6.1 was used (Cruz, 2016). Validation of the tetrazolium test was done by multiple comparison of the viability means of the six treatments plus the average viability, obtained with the conventional germination chamber germination test, as an additional treatment.
RESULTS AND DISCUSSION
External and internal morphology of seeds. The morphometric characteristics of the S. parahyba seeds (Table 1) showed minimal variability for the variables seed width (AS), average of 1,32 ± 0,024cm; seed length (LS), average of 2,09 ± 0,043cm and the AS/LS ratio (RALA), 0,64 ± 0,031, as a reflection of its high homogeneity, which is explained by the reduced variance and low coefficient of variation, which contrast with those reported by Fontana et al. (2015), in Prosopia alba, in which the same variables registered significant variation.
Table 1 Descriptive statistics for the biometric characteristics of Schizolobium parahyba seeds in the department of Córdoba (Colombia).
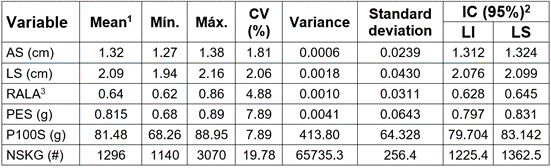
1: Mean of 500 data; Min. = Minimum value, Max. = Maximum value; CV: Coefficient of variation,2: IC (95%): Confidence interval at 5% probability; LI: lower limit; LS: upper limit; 3: AS: Seed width; LS: seed length; RALA: AS / LS ratio (Dimensional): P100S: Fresh weight of 100 seeds; NSKG: Number of seeds / kilogram.
The weight of one seed (PES) of 0,815 ± 0,064g, the weight of 100 seeds (P100S) of 81,48 ± 64,33g and the average number of seeds / kg (NSKG) was 1296 ± 256,4 (Table 1), showed greater heterogeneity reflected in their coefficients of variation, a result similar to those reported by Ferreira et al. (2007) and Da Silva & Vitti (2008), in the species Clitoria fairchildiana, also of the Fabaceae family, which is a function of its reproductive capacity associated with genetic and environmental effects, in which the genetic diversity of the population stands out, due to cross-pollination and its biotic and abiotic pollinating agents (Mendizábal-Hernández et al. 2013), which allows differentiating species belonging to the same genus.
Regarding their external morphology, the seeds are oval, flattened testa or smooth, hard and shiny seed coat, with a rounded apex (micropyle and hilium) and a brown, attenuated base with a darker edge (Figure 1 a). Its internal anatomy is composed of radicle, epicotyl and cotyledons, of glassy consistency, which when hydrated is gelatinous, viscous and transparent. The embryo is classified as axial type, straight, occupies all the seed, the cotyledons are apple green, fleshy and smooth (Figura 1 b). These characteristics are consistent with those reported for this same species, by Ferreira et al. (2007), Fogaça et al. (2011) and Freire et al. (2015).

Figure 1 General description of the morphology: (a) external and (b) internal, of the seed of Schizolobium parahyba.
Determination of topological patterns. The staining patterns identified in the S. parahyba seeds are described and shown graphically in figure 2. They are similar to those reported in S. parahyba, by Ferreira et al. (2007) and Fogaça et al. (2011) and in other tree species, such as Parkia multijuga (Costa et al. 2018), Libidibia ferrea (Costa-Carvalho et al. 2017), B. quinata and A. excelsum (Espitia et al. 2017 a), Poincianella pyramidalis (Macedo-Sousa et al. 2017), C. odorata and C. pyriformis (Espitia et al. 2017 b) and Crambe marítima (Guimarães et al. 2015).
The seeds recorded variations in the intensity of staining, because the tetrazolium solution makes it possible to determine alterations of seed tissues (ISTA, 2014; Lima et al. 2010), producing in living tissues triphenil formazan, that identifies the respiratory activity of the mitochondria and, as a result, shows that there is cell viability. Therefore, the red color in the embryos is a positive indicator of the viability of the seeds (Craviotto et al. 2008) and usefulness in seed quality control programs. Those faintly colored regions in some parts of the embryo indicate that the cells have decreased respiratory activity and, consequently, less activity of dehydrogenase enzymes (Rodríguez et al. 2008; Rao et al. 2007).
Based on the characteristics observed in the embryos, the seeds were classified into four viable classes (1 and 2), doubtful and inviable, as shown in figure 2.
Tetrazolium concentrations and staining times to measure seed viability. The analysis of variance in topological patterns and the results of the means separation tests are reported in table 2. In S. parahyba seeds, the treatments presented significant differences (p <0.05), in the viable (V), Inviable (I) and total viability (VT) categories, evidencing that tetrazolium concentrations and staining times, affected estimation of the effect, in terms of coloration of essential seed tissues, the opposite being the effect on doubtful seeds (D). Similar results have been reported in S. parahyba, by Ferreira et al. (2007) and Fogaça et al. (2011) and in other tree species, such as B. quinata and A. excelsum (Espitia et al. 2017 a) and C. odorata and C. pyriformis (Espitia et al. 2017 b).
Table 2 Values for viability and germination (%) of Schizolobium parahyba seeds, in the tetrazolium test and the germination test.
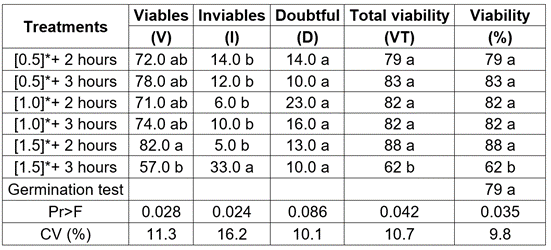
* Concentration of 2. 3. 5 tetrazolium chloride (%); the letters indicate significant differences according to Duncan's test at 5%. CV = Coefficient of variation.
When examining the viability percentage (Table 2) and the quality of the staining per treatment (Figure 3), it can be seen that the statistical differences between the treatments were fundamentally defined by the treatment of 1.5% tetrazolium concentration with immersion of three (3) hours, since it statistically originated the lowest percentage of estimation of viable, inviable and total viability seeds. It can be seen that the differences in staining, at a qualitative level, are not very marked in the rest of the treatments, suggesting that the combinations allow a rapid, clear and reliable observation of the stain in the living tissues of the seed and, therefore, of the viability, according to what has been reported in several studies on forest seeds (Costa et al. 2018; Costa-Carvalho et al. 2017; Espitia et al. 2017 a; 2017 b; Abbade & Massanori, 2014; ISTA, 2014; Fogaça et al. 2011).
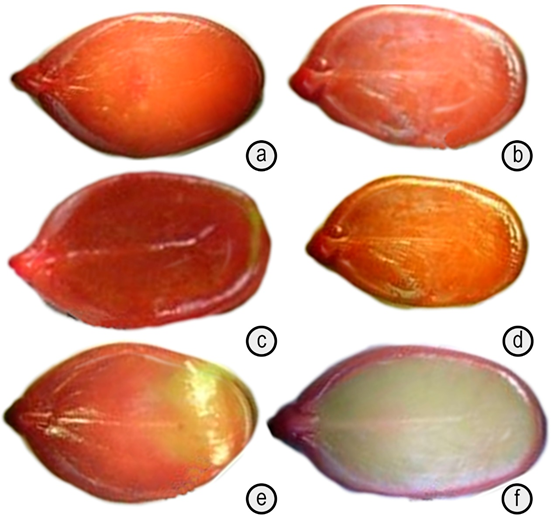
Figure 3 Images of seed viability in the tetrazolium test on Schizolobium parahyba seeds. a. 0,5%, 2hours; b. 0,5%, 3hours; c. 1%, 2hours; d. 1% 3hours; e. 1,5%, 2hours; f. 1,5%, 3hours.
These results allow us to infer that the use of any of the concentrations of 0.5% or 1.0% of tetrazolium, with times of 2 or 3 hours of immersion, is efficient and reliable to determine the viability of the seeds of this species. Therefore, using tetrazolium chloride solution at concentrations of 0.5% for 2 hours of immersion, is the most economical and viable alternative, allowing adequate staining of seed tissues, without impairing the visualization of viability. Additionally, the first two hours of water absorption by the seed are important, since they are related to the enzymatic activity and, therefore, to the final coloration (Lima et al. 2010). Similar results to those found in this study have been reported in the species Parkia multijuga (Costa et al. 2018), Libidibia ferrea (Costa-Carvalho et al. 2017), B. quinata and A. excelsum (Espitia et al. 2017 a), Poincianella pyramidalis (Macedo-Sousa et al. 2017), Copaifera langsdorffii and Schizolobium parahyba (Fogaça et al. 2011).
The result obtained in the germination test, with 79% of normal seedlings, did not present a significant difference with the percentages of viable seeds, estimated by the different treatments of the tetrazolium biochemical test (Table 2). This result suggests that the S. parahyba seeds used in the study did not show dormancy effect, under the controlled optimal conditions of the laboratory, where the research was carried out and is consistent with those reported in ahuyama, by Barros et al. (2005) and in cucumber by Lima et al. (2010). The presence of inviable seeds is due to the degradation of cell membranes by lipid peroxidation and non-enzymatic peroxidation, which are factors that contribute to the degradation of seed viability (Ravikumar et al. 2002).
In conclusion, the morphometric characteristics related to the seed width, seed length and seed width / length ratio showed little variation, but not the weight of one seed, weight of 100 seeds and number of seeds per kilogram, derived from the genetic and environmental effects. The tetrazolium test and conventional germination did not register significant differences, therefore the concentration of 0.5% tetrazolium plus 2 hours of immersion is reliable to estimate the viability of seeds.