INTRODUCTION
Microcrambus Błeszyński is the largest genus of Crambinae (Pyralidae sensu lato) in the New World, with 53 recognized species (Nuss et al. 2020), including the species formerly placed in MicrocramboidesBłeszyński, 1967 and TortriculladiaBłeszyński, 1967, following the results of the phylogenetic analyses of Léger et al. (2019). Except for Landry (1995), who provided a general review of the genus, and Munroe (1995) and Landry in Nuss et al. (2020), who listed all of the known species, there has been no taxonomic treatment on Microcrambus since the works of Błeszyński (1963, 1966, 1967) and Klots (1968). This may be due to the absence of species of economic importance in the genus (Landry, 1995) as well as the difficulty in tackling a large group of small species mostly resembling each other. Genus Microcrambus as well as half of its Neotropical species were described by Błeszyński (1963, 1967) and a synapomorphy for the genus, i.e. the ventro-apically produced phallus, was presented by Landry (1995). The Neotropical fauna of Microcrambus consists of 47 species, but only three species have been recorded from Colombia previously, as shown below. Based on the specimens collected in 2018, by BL in Colombia, two new species are described and three additional species are recorded here from Colombia for the first time.
MATERIAL AND METHODS
The specimens treated here were collected by BL in Colombia between July 18 and August 8, 2018 with the macro permit to collect insect specimens without commercial purposes of the “Universidad Nacional de Colombia”. They were studied in the “Muséum d'histoire naturelle” in Geneva (MHNG) with the export permit issued by the “Asociación Nacional de Licencias Ambientales-ANLA”. Two localities were visited, i.e. “Parque Natural Chicaque,” Cundinamarca Department, a few kilometres west of Bogotá, where sampling was performed between 2100 and 2250m, and forests around Leticia, in Amazonas Department, around 80m in elevation. The specimens were attracted to light using Gunnar Brehm’s fantastic Lepiled (Brehm, 2017) in a 2m high tower of white gauze set usually on the ground, but one evening on a platform in a tree, at 35m above ground level.
Specimens were dissected in 30% ethanol following maceration in KOH at 60°C for one hour. Dissected parts were stored temporarily in lactic acid stained with Orange G, thus allowing their description in three dimensions. Mounting followed the method given by Landry (1995) except that xylol and Canada balsam were respectively replaced by Euparal essence and Euparal.
Photos were taken with a Leica M205, a Leica DFC425 camera and its associated imaging software. The photos were stacked using Zerene Stacker of Zerene Systems LLC and minimally modified using Adobe Photoshop Elements.
The labels of the holotypes are transcribed exactly, with vertical bars to represent changes of lines and missing letters of abbreviated words placed in square brackets. For the paratypes, the presentation of the label data is simplified, with the missing letters of abbreviated words placed in square brackets only on their first occurrence.
A total of 18 specimens were processed for DNA barcoding of the cytochrome c oxidase I (COI) mitochondrial marker in the MHNG. DNA was extracted from specimens’ legs using the commercial Chelex resin in deionised water containing 0.1mg/mL proteinase K. A fragment of 658 bases of the COI gene was amplified using the versatile invertebrate primers (5’-AGT TCT AAT CAT AAR GAT ATY GG -3’) and (5’- TAA ACT TCA GGG TGA CCA AAA AAT CA -3’) (Nadler et al. 2006). PCR reactions were carried in a 20μL reaction volume, including 2x MyFi Mix (containing the polymerase, dNTPs and salts), 4μM of each primer, and 4μL of extracted DNA. The thermocycling program followed Blasco-Costa et al. (2016): it started with an initial denaturation at 95°C for 2 min, followed by 40 cycles with 40 s denaturation at 94°C, 30 s annealing at 50°C and 45 s extension at 72°C, and ended by a final extension for 5 min at 72°C. PCR products were visually inspected by electrophoresis on an agarose gel and purified using Exo-SAP enzymes (Werle et al. 1994). Sequencing was carried for both strands with the same primers used for the amplification. Sequences were aligned and visually checked for errors using the software Geneious 8.1.9 (https://www.geneious.com). Assembled sequences and trace files, associated to collection data and pictures of the specimens, were deposited for all individuals on the BOLD platform (http://www.boldsystems.org) under accession numbers LEPBL001-19 to LEPBL018-19. A taxon ID tree of all sequences available to us as of April 2020 was constructed using tools on BOLD. A total of 428 COI sequences of Microcrambus (including those deposited as Tortriculladia) were aligned using the BOLD amino-acid based aligner, and a neighbour-joining tree based on Kimura 2-parameter distances (K2P) was constructed (raw file deposited on Zenodo doi: https://doi.org/10.5281/zenodo.3760713).
The following abbreviations are used: ‘BL’ for ‘Bernard Landry’, ‘leg.’ for ‘legit’, ‘m’ for ‘meters’, ‘UNAB’ for ‘Universidad Nacional Agronomía Bogotá, Museo Entomológico de la Facultad de Ciencias Agrarias de la Universidad Nacional de Colombia’, ‘MHNG’ for ‘Muséum d’histoire naturelle, Genève, Switzerland’, and ‘ZMB’ for ‘Museum für Naturkunde Berlin, Leibniz-Institut für Evolutions- und Biodiversitaetsforschung, Berlin, Germany’.
The species collected in Colombia were identified with the original descriptions in addition to the publications of Błeszyński (1963, 1967) as well as with photos of the type specimens of all Neotropical species made by BL over the years during museum visits or following requests of photos to curators. In the case of M. expansellus (Zeller, 1877) (type locality: Panama, Chiriqui), only the illustrated original description is presently useful as the holotype, the single known specimen, deposited in the ZMB, is damaged beyond recognition, having lost its forewings, one hindwing, several legs, the labial palpi (as mentioned in the description), one antenna entirely and the other partly, and the whole abdomen.
RESULTS AND DISCUSSION
Taxonomic section:
Microcrambus arevaloi Landry, sp. n.
Material examined: 3 ♂, 3 ♀ from Colombia, Amazonas. Holotype: ♂, ‘COLOMBIA, Amazonas, Leticia, Reserva Omagua, at LepiLED | 4°07’37’’S, 69°56’60’’W | 115m elev(ation)., on tree platform at | 35m above ground, 4.viii.2018 | leg. B. Landry, E. Ospina’; ‘If selected as holotype | return to Museo | Entomológico, UNAB | Bogotá, Colombia’; ‘(genitalia slide) BL 1861 ♂’; ‘DNA voucher | Lepidoptera | B. Landry, n° 00005’; ‘HOLOTYPE | Microcrambus arevaloi B. Landry’. Deposited in UNAB. Paratypes: 2 ♂, 3 ♀. 1 ♂ with same data as holotype except DNA voucher Lepidoptera B. Landry n° 00007; 1 ♀ with same data as holotype except genitalia slide (and database) n° MHNG-ENTO 84603 ♀ and DNA voucher Lepidoptera B. Landry n° 00010; 1 ♀ with same data as holotype except DNA voucher Lepidoptera B. Landry n° 00009; 1 ♂ from Leticia, Univ(ersidad). Nacional de Colombia, 4°11’37’’S, 69°56’23’’W, 80m elev(ation)., 24, 25, 31.vii.2018, at lights (B. Landry, H. Arévalo, F. Serna, J. De Prins), DNA voucher Lepidoptera B. Landry n° 00006; 1 ♀, Leticia, Reserva Natural La Manigua, 4°04’38’’S, 69°59’56’’, 80m elev., 26, 27, 30.vii., 2.viii.2018, at lights (B. Landry, H. Arévalo, F. Serna, J. De Prins), DNA voucher Lepidoptera B. Landry n° 00008. Deposited in UNAB and MHNG.
Etymology: In honour of Helber Adrian Arévalo Maldonado, Ph.D. candidate at Universidad Nacional de Colombia, Bogotá, for his friendship, hospitality and for securing funds and collecting permits for BL in Colombia.
Diagnosis: Among the 40 known similarly coloured Microcrambus species, the forewing pattern can be distinguished by the reduction of the median markings to a few mostly faint spots seemingly aligned in two well separated diagonal lines running from the dorsal margin submedially towards the apex, the two distinct and clean dark brown subterminal lines bent towards the base before the costa and abutting a small but clear dark brown triangle on costa subapically, and a slightly darker band along the costa from the base until the subterminal lines (Figures 1 a, b). In these aspects the species is similar to M. matheriKlots, 1968, for example, but although the male valva of M. matheri also has a long costal arm, the latter is terminally rounded whereas that of M. arevaloi ends in a pair of spine-like projections. Microcrambus caracasellusBłeszyński, 1967 and M. discludellus (Möschler, 1890) also have a long free costal arm on the male valva but in both cases it is simple and terminally pointed. None of the other pale brown species of Microcrambus have a long and free costal arm of the valva as far as known, and, in species for which the male is unknown, the habitus lacks a pair of clean (without associated projections of the basal subterminal line) subterminal lines and well contrasted median markings.

Figure 1 Specimens of Microcrambus species. (a-b) Microcrambus arevaloi; a. Male holotype, UNAB; b. Largest female paratype, MHNG. (c-d) Microcrambus leticiensis; c. Male holotype, UNAB; d. Female paratype, MHNG.
CO1 barcode of holotype: 5‘-GACATTATATTTTATTTTCGGAATCTGAGCAGGAATAGTAGGTACATCTTTAAGACTTTTAATTCGTGCTGAATTAGGTAATCCAGGATCTTTAATTGGTGATGACCAAATCTACAATACTATTGTCACAGCTCATGCTTTTATTATAATTTTTTTTATAGTTATACCAATTATAATTGGAGGATTTGGAAATTGATTAGTTCCTTTAATATTAGGAGCTCCAGACATAGCTTTCCCACGAATAAATAATATAAGATTTTGATTATTACCCCCTTCTTTAACATTATTAATTTCTAGAAGAATTGTAGAAAATGGAGCTGGGACAGGATGAACGGTTTACCCCCCCCTTTCATCTAATATTGCTCATGGAGGAAGATCAGTAGATCTTGCTATTTTTTCATTACATTTAGCTGGAATTTCTTCAATTTTAGGAGCTATTAATTTTATCACAACAATCATTAATATACGAATTAATAATTTATCCTTTGATCAAATACCATTATTTGTTTGATCAGTAGGAATTACTGCTTTACTTTTACTTCTATCACTACCAGTTTTAGCGGGAGCTATTACTATACTTTTAACTGATCGAAATTTAAATACCTCTTTCTTTGACCCTGCGGGAGGAGGAGATCCAATTTTATATCAACATTTATTT-3’
Description: MALE (n=3) (Figure 1 a). Head with frons slightly rounded although not projecting; vestiture short scaled and appressed except for porrect tuft of slightly longer scales laterally in front of antennal base, and fan of mostly short scales directed medially behind antenna and eye margin; colour mostly white to pale whitish cream with light greyish brown as a small patch at base of antenna frontomedially, in middle of frons, and on lateral tuft. Antenna light whitish cream on most of scape; apex of scape, pedicel, and flagellomeres greyish brown with few dirty white scales laterally on basal flagellomeres. Maxillary palpus white at base, followed by brown patch at base of longer, projecting apical scales mostly white with some whitish cream and greyish brown. Labial palpus white on two basal segments, brown (except medially) at apex of first segment dorsally, in middle of 2nd segment, and at base of 3rd, pale whitish cream apically. Haustellum pale cream. Thorax mostly white to pale whitish cream, blackish brown at base of tegula and greyish brown to brown at tip of patagium and tegula, and tip of mesoscutellum. Forewing length: 5.5-5.75mm (holotype: 5.75mm); wingspan: 12.0-12.5mm (holotype: 12.5mm). Wings with colour and pattern as illustrated (Figure 1 a) and diagnosed above. Prothoracic leg coxa and trochanter white to pale whitish cream; femur and tibia greyish brown dorsally, ventrally, including epiphysis, cream; tarsomeres darker, uniformly blackish brown. Mesothoracic leg coxa and trochanter as on foreleg; femur pale whitish cream with grey apex; tibia dorsally grey with whitish cream apex, ventrally whitish cream, with spurs darker, blackish brown; tarsomeres greyish brown to blackish brown, darkening toward distitarsus, with white to whitish cream apex on tarsomeres I-IV. Metathoracic leg coxa and trochanter as on foreleg; femur, tibia, and first tarsomere whitish cream; tarsomeres II-V as on foreleg. Abdomen whitish cream dorsally, white ventrally. Intersegmental membrane VII-VIII around genitalia set with thin long scales about as long as valva for longest of them.
Male genitalia (n=1) (Figures 2 a, b). Uncus of moderate girth and length, abundantly setose dorsally and laterally, ending in short point bent downward. Gnathos arms and terminal section thin, latter slightly shorter than uncus, with apex bent downward. Tegumen of medium size, with dorsal connection slightly longer than uncus. Juxta a pair of rectangular plates shortly connected at base and subapically, scobinated ventromedially at base. Valva with cucullus narrow, narrowing from base of costal arm to narrowly rounded apex, with low medioventral hump before base of costal arm more thickly sclerotized and setose; costal arm thickly sclerotized, running along cucullus and almost reaching its tip, about equal in girth from base to more abundantly setose apex adorned with two spine-like projections: one very short pointing dorsally and other twice as long directed apicoventrally. Vinculum short, with more thickly sclerotized lateral and ventral margins, laterally expanded to about twice median length. Pseudosaccus small, pear shaped in dorsal view. Phallus short and rather bulky, about as long as valva, with digit-like coecum penis slightly longer and wider than posterior section of shaft, with short projecting subapical plate ventrally not reaching apex of shaft, latter with more thickly sclerotized narrow ventral wall set with minute spikes ventrally; vesica adorned with eight cornuti of medium size and large scobinated section apically in invaginated condition.
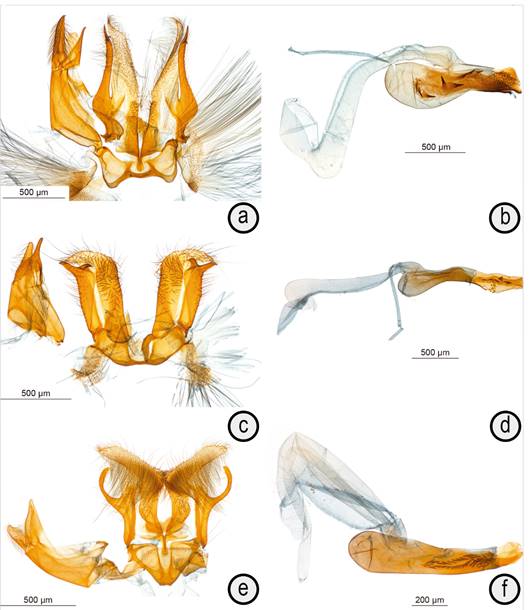
Figure 2 Male genitalia of Microcrambus species, left showing the genitalia without phallus and right the phallus; a and b. Microcrambus arevaloi holotype; c and d. Microcrambus leticiensis holotype; e and f. Microcrambus elpenor, slide BL 1858, Leticia, Reserva Natural La Manigua.
FEMALE (n=3) (Figure 1 b): Forewing length: 5.5-6.5mm (wingspan: 12.0-14.0mm). Frenulum with 2 acanthae. Hindwing not darker than that of male. Abdominal segment VII about 1/3 longer than segment VI, slightly more thickly sclerotized distally and more densely scaled.
Female genitalia (n=1) (Figure 3 a). Papillae anales lightly sclerotized, with modest setation and broad coverage of spinules, with posterior margin only lightly concave. Posterior apophyses straight, short, about as long as papillae anales and 2/3 as long as their width, bulging medially along about 2/3 of length, ending in thin point. Tergite VIII of medium width, enlarging lateroventrally into quadrangular sections about twice as long as dorsomedially, covered with spinules, and bent at right angle to form more thickly sclerotized dorsal plate of sterigma also spinulose and fused to even more thickly sclerotized ventrally disconnected ring around base of wide, lightly sclerotized protruding tube folded 3X upon itself and spinulose on distal 1/3 ventrally and on distal 2/3 dorsally; ostial margin of tube symmetrical, without ornamentation except for spinules; sclerotized tube internally more thickly sclerotized, long, reaching abdominal segment V, slightly curved, with half-ringed sclerotized inception of ductus seminalis on right side at about 1/3 from base of tube. Ductus bursae slightly shorter than internal section of tube, lightly scobinated near corpus bursae. Corpus bursae circular, small, about as long as ductus bursae, lightly scobinated all over; unique signum near proximal end on right side, a wide scobinated folded plate about 1/3 as wide as corpus bursae and with fold reaching inside corpus bursae to about 1/4 of its width.
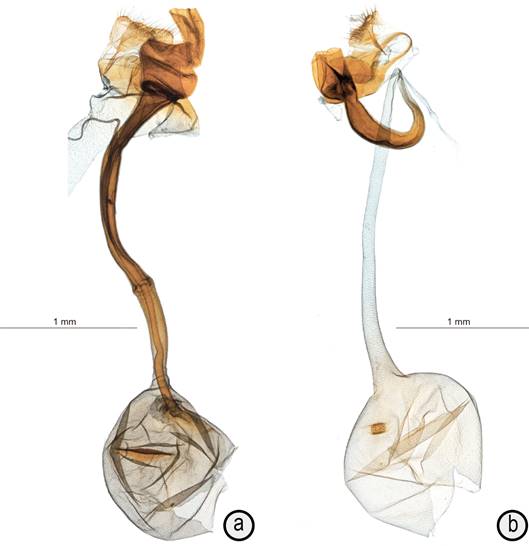
Figure 3 Female genitalia of Microcrambus species. A) Microcrambus arevaloi paratype, with tubular section of spermatophore left inside ductus bursae, slide MHNG-ENTO- 84603; b) Microcrambus leticiensis paratype, slide MHNG-ENTO-84602.
Biology: Unknown except that adults come to light and fly low above the ground as well as at 35m above, in the tree canopy.
Distribution: Presently known only from the Amazonas Department of Colombia, near its capital, Leticia.
Remarks: The six available specimens were sampled for their DNA and yielded full sequences of the COI barcode. In the neighbour-joining tree, the sequences of M. arevaloi cluster with a group of species including M. jolasBłeszyński, 1967, M. elegans (Clemens, 1860) and M. polingi (Kearfott, 1908) with a minimal K2P divergence of 8.4%. Four of the specimens (LEPBL005, 007, 008, 009) have the same barcode sequence, one specimen (LEPBL006) diverges by one transition (K2P 0.2%) from the foursome and specimen LEPBL010 diverges by three other transitions (K2P 0.5%) from the foursome. The six specimens available are each missing one-two metathoracic legs, retrieved for DNA barcoding. The colouration of the abdomen is based only on the undissected paratypes as the holotype had been dissected prior to description. Four of the six specimens were collected on a platform at 35m above ground and during about two hours, beginning at sunset, more than 200 species of moths were observed or collected there, the most fruitful collecting event of the 12 performed at Leticia by BL in 2018.
Microcrambus leticiensis Landry, sp. n.
Material examined: 1 ♂, 1 ♀. Holotype: ♂, ‘COLOMBIA, Amazonas, Leticia | Reserva Natural La Manigua | at lights, 80m elev(ation)., 4°04’38’’S | 69°59’56’’W, 26, 27, 30.vii., | 2.viii.2018, leg. B. Landry, H.| Arévalo, F. Serna, J. De Prins’; ‘If selected as holotype | return to Museo | Entomológico, UNAB | Bogotá, Colombia’; ‘(genitalia slide) BL 1860 ♂’; ‘DNA voucher | Lepidoptera | B. Landry, n° 000011’; ‘HOLOTYPE | Microcrambus | leticiensis | B. Landry’. Deposited in UNAB.
Paratype: 1 ♀. Same data as holotype except genitalia slide (and database) n° MHNG-ENTO 84602 ♀ and DNA voucher Lepidoptera B. Landry n° 00012. Deposited in MHNG.
Etymology: Derived from the name of the type locality, the municipality of Leticia, Colombia, situated alongside the Amazon River.
Diagnosis: Among the 40 described species of Microcrambus with a pale brown or greyish brown background colour, this one can be recognized by the generally darker greyish brown colour, appearing mouse grey, with darker markings in a pair of spots submedially on dorsal half, but not touching the dorsal margin, a short, submedian costal bar directed toward the tornus, a median line from the middle of the dorsal margin, directed towards the apex and veering back at right angle towards the costa and reaching it, a straight and clean (without associated projections) subterminal black line slightly wider on costal half followed by a paler line, a finer dark line reaching the tornus, and a white section with contrasting dark brown spots between veins on the terminal margin. In male genitalia (Figures 2 c, d) , this species is most similar to M. bellargusBłeszyński, 1967 but with the uncus shorter than the gnathos as opposed to equal in length in M. bellargus, the sclerotized valval costa ending in two separate projections as opposed to one in M. bellargus, the absence of a short rounded projection medially on the sacculus subapically, the shorter and less slender phallus, and the vesica with a row of six short spine-like cornuti along with several smaller, similarly shaped cornuti mostly ventrally toward the apex of the shaft in its invaginated state, as opposed to the absence of cornuti on the vesica of M. bellargus. In female genitalia (Figure 3 b) this species is very similar to M. castrellus (Schaus, 1922), known from the single female holotype, but the internal tube of the antrum is shorter and apparently not folding onto itself completely in M. castrellus and the ductus bursae is shorter, i.e. only 1.5 X the length of the corpus bursae whereas is it about 2.2 times this length in M. leticiensis. In habitus the holotype of M. castrellus differs more conspicuously than in genitalia, with a paler ground colour with faint warmer-brown (described as sayal brown) markings, including an incomplete basal subterminal line made of several fine striated lines without a contrastingly paler line before the second subterminal line.
CO1 barcode of holotype: 5’-AACTTTATATTTTATTTTTGGAATTTGAGCAGGAATAATCGGAACTTCTTTAAGTTTATTAATTCGTGCTGAATTAGGTAACCCTGGATTTTTAATTGGAGATGATCAAATTTATAATACTATTGTTACAGCTCATGCTTTTATTATAATTTTTTTTATAGTAATACCAATTATAATTGGAGGTTTTGGAAATTGATTAGTTCCTTTAATATTAGGAGCCCCAGATATAGCTTTCCCACGAATAAATAATATAAGATTTTGATTACTCCCCCCATCTCTGACTCTATTAATTTCTAGAAGAATTGTCGAAAATGGAGCAGGAACAGGATGAACAGTTTACCCCCCACTTTCATCTAATATTGCTCATGGAGGTAGATCAGTTGATCTAGCCATTTTTTCCCTTCACTTAGCTGGGATTTCTTCAATCTTAGGAGCCATTAATTTTATTACTACAATTATTAATATACGAATTAATGGATTAATATTTGACCAAATACCTTTATTTGTTTGATCTGTAGGAATTACTGCTTTATTACTATTATTATCTTTACCTGTATTAGCGGGAGCTATTACTATACTTTTAACAGATCGAAATTTAAATACTTCTTTCTTTGACCCTGCTGGAGGAGGAGATCCAATTTTATATCAACATTTATTT-3’
Description: MALE (n=1) (Figure 1 c). Head with frons rounded, not projected; vestiture partly missing in unique specimen, dirty white to greyish brown, darker greyish brown at eye margin posteriorly and dorsomedially projecting fan behind antenna. Antenna dark greyish brown with dirty white ventrally on scape and pedicel. Maxillary palpus greyish brown with dirty white apically. Labial palpus mostly greyish brown laterally, darker on first segment, white medially, dirty white at apex of second and third segments. Haustellum dark greyish brown dorsally, dirty white laterally. Thorax with patagium scales greyish brown medially, dirty white laterally; tegula pale greyish brown (but base not visible); tip of mesoscutellum dark brown; metascutum white with pale greyish brown medially. Wings with colour and pattern as illustrated (Figure 1 c) and diagnosed above. Forewing length: 5.0mm (wingspan: 11.0mm). Prothoracic leg coxa and trochanter dirty white; femur pale greyish brown ventrally, not hidden dorsally; tibia and tarsi dark greyish brown. Mesothoracic leg coxa and trochanter dirty white; femur dirty white; tibia greyish brown, darker at base and on spurs; basal tarsal segment pale greyish brown; tarsal segments II-IV dark greyish brown with pale greyish brown tip; distitarsus all dark greyish brown. Intersegmental membrane VII-VIII medioventrally devoid of scales but with sclerotized finger-tip shaped plate about as long as pseudosaccus; lateroventrally with narrow scaled area enlarging, folded, with narrow scales of medium length; laterally with enlarged, rounded scaled area with long hair-like scales.
Male genitalia (n=1) (Figure 2 c, d). Uncus of medium girth and length, moderately setose, apically narrowly rounded. Gnathos narrow, slightly longer than uncus, with distal section gently curving downward, with short apical hook. Tegumen of medium size, with pedunculi medially about as wide as dorsal connection. Juxta of medium size, heater-shield shaped with short and rounded apicolateral projections. Valva with thickly sclerotized sacculus, without ornamentation but with coat of short, stiff setae medially; cucullus directed upward at right angle, short, with pointed apex; costa of valva thickly sclerotized, ending before cucullus in pair of short hooks: ventral directed medially at right angle, dorsal directed dorsomedially at about 50°. Vinculum quadrangular, with wide, straight basal margin. Pseudosaccus long and thin, banana-shaped, concave dorsally. Phallus of medium length and girth, as long as vinculum+valva, slightly down-curved, with short coecum penis, with ventral margin forming thin point shortly produced at apex; vesica with six short spine-like cornuti, increasing in size towards apex in invaginated condition, also with several smaller, similarly shaped cornuti mostly ventrally toward apex of shaft in its invaginated state, and wide coverage of scobination.
FEMALE (n=1) (Figure 1 d): Forewing length: 5.5mm (wingspan: 12.0mm). Frenulum with 2 acanthae. Hindwing not darker than that of male. Tergite VII longer than VI, with posterior half more thickly sclerotized, apically set with more robust scales of mostly medium length and variable width, the longer the thinner, and slightly curved ventrally towards their apices.
Female genitalia (n=1) (Figure 3 b). Papillae anales triangular with almost straight posterior margin, with moderate setation and wide coverage of spinules; posterior apophyses straight and short, about as long as papillae anales and half as long as their width. Tergite VIII a very narrow band expanding lateroventrally into pear-shaped plates with medioventral connection about half as wide as widest section. Sterigma a pair of medioventrally unconnected lateroventral oblong plates of medium size at right angle from longitudinal plane of abdomen (thus directed ventrally); plates medially abutting diagonally constricted thickly sclerotized tube expanding into asymmetrical exterior section with left side straight and right side bulging at base; ostial margin of tube symmetrical, without ornamentation or spinules; tubular internal section of sterigma narrow, about half as wide as external section, curving back onto itself at 180° and narrowing to about 1/3 basal section distally. Ductus seminalis at distal end of sclerotized tube, at base of ductus bursae. Ductus bursae thin, only slightly and evenly expanding before reaching corpus bursae, and long, reaching apical margin of third abdominal segment and 2.2 X length of corpus bursae, scobinated lightly all along. Corpus bursae small, circular, lightly scobinated all over; unique signum near proximal end on right side, a folded circular plate with thick scobination.
Biology: Unknown except that adults come to light and fly in July and August.
Distribution: Colombia, Amazonas Department, around Leticia.
Remarks: The two specimens were sampled for their DNA and both yielded the same full sequences of the COI barcode. In the neighbour-joining tree, the sequences of M. leticiensis cluster with a sequence of a specimen identified as Microcrambus croesusBłeszyński, 1967, from Texas with a divergence of 9.0%. The two specimens available are each missing both metathoracic legs, retrieved for DNA barcoding. The colouration of the abdomen was not recorded as both specimens had been dissected prior to description.
Toward a list of the species of Microcrambus Błeszyński in Colombia
Microcrambus arevaloi, sp. n. described above from Amazonas, Leticia.
Microcrambus discludellus (Möschler, 1890). Occurrence based on type locality (Colombia, Tolima, Honda) of Crambus micralisHampson, 1919, a synonym of M. discludellus, which was itself described from Puerto Rico. Crambus domingellusSchaus, 1922, another synonym was described from Dominican Republic. The species is distributed also in the USA (Florida, South Carolina) (Moth Photographers Group, 2020).
Microcrambus elpenorBłeszyński, 1967. Six male and two female specimens were collected around Leticia by BL in 2018. Three of the males and one female were barcoded and show the exact same barcodes. The dissected female have genitalia matching perfectly with the illustration provided by Błeszyński (1967, figure 59) whereas a dissected male, illustrated here (Figures 2 e, f) have genitalia that differ slightly from the illustration provided by Błeszyński (1967, figure 63) in the slightly shorter and wider uncus, the slightly longer dorsal projection of the valva and the slightly shorter and wider valva with the terminal section directed dorsally at an angle closer to 90°. Described from Chiapas, Mexico (type locality) as well as Guyana and Trinidad (Trinidad and Tobago), the species is also known to occur in Brazil, Bahía (Camacan and Porto Seguro) (specimens collected by BL and Vitor Becker and identified by BL).
Microcrambus immunellus (Zeller, 1872). Occurrence based on type locality (Colombia, Cundinamarca, near Ubaque). Records of other localities by Zeller (1877, 1881) are preferable to other species according to Błeszyński (1967). Druce (1896: 290) mentions it from Costa Rica, although this record has not been validated with dissections.
Microcrambus jolasBłeszyński, 1967. Based on two males collected around Leticia by BL in 2018. One dissected male has genitalia matching perfectly with the illustration provided by Błeszyński (1967, figure 72). Described from Mexico, Chiapas, the species is also known to occur in south west Nicaragua from specimens collected by BL that show a 2.8-3.5% divergence in CO1 barcodes from the two Colombian specimens.
Microcrambus leticiensis, sp. n. described above from Amazonas, Leticia.
Microcrambus mercuryBłeszyński, 1963. Occurrence based on type locality (Colombia, Chocó Department, Condoto). Described from two males, this species has not been reported since.
Microcrambus cf. mixena (Błeszyński, 1967). This record is based on the two females collected by BL around Leticia in 2018. Both were barcoded and belong to the same BIN (Barcode Index Number) as three specimens collected in French Guiana (BOLD: AAU7498). These three specimens were borrowed from the “Muséum national d’histoire naturelle” (Paris, France) and one of them, a male, was dissected. The genitalia of this male match reasonably well with the illustration provided by Błeszyński (1967, fig. 42) for his M. mixena, a species described from Peru, near Aguaytia, at 400m in elevation, on the Eastern slope of the Andes, about 825km from Leticia as the crow flies. A photo of the unique holotype of M. mixena, deposited in the Canadian National Collection of Insects, Ottawa, Ontario, Canada, is also a good match in habitus with the Leticia specimens although three other species are similar in habitus. A study of the genitalia preparation of the holotype of M. mixena and a more in-depth taxonomic revision of this group of six species formerly placed in Tortriculladia Błeszyński, will be necessary to confirm the identity of the Colombian specimens at hand.
Final remarks
The Colombian fauna of Microcrambus is shown here to consist of eight recorded species. This is clearly an incomplete assessment of this fauna given the large number of species of Microcrambus and paucity of previous collecting efforts on micro moths in Colombia. Specimens of Microcrambus from Colombia probably exist in some natural history collections, but they were not requested here for the purposes of this project. Specimens in the field should be looked for in the lowlands especially, as the genus doesn’t appear to be common at high elevations as it was not recorded during BL’s collecting efforts at Parque Natural Chicaque (2100-2250m) nor in the páramo of the country by Wolfram Mey (ZMB), in 2017.















