INTRODUCTION
The Andes are a range of mountains with a length of 8,500km that extends from Chile, northern Argentina, Bolivia, Peru, Ecuador, and Colombia to Venezuela, with an average altitude between 3,000 and 4,000m a.s.l., bordering the coast of the Pacific Ocean (Guerrero et al. 2011), with a width that varies between 250 and 750km and occupying an area of about 2,870,000km2 (Orme, 2007). Having 15% of the total richness of the world's plants, the tropical Andes represent one of the main key points of the planet biodiversity (Peyre et al. 2019).
Fruit species originate in the Andes, known as “exotic” ones in temperate zones and having a great potential for national producers and consumers and, furthermore, are exported in significant quantities (Moreno-Miranda et al. 2019; National Research Council, 1989). These fruits represent an important part in the diet of the Andean population and are increasingly found in specialized markets (Acosta-Quezada et al. 2015).
Many fruit trees have their origin in the Andean areas (Ligarreto, 2012), especially numerous Solanaceous species (Blancke, 2016; Criollo et al. 2017). Solanaceae is one of the main families of economically important plants in Colombia (Torres-González, 2019; Almanza-Merchán et al. 2016). These are the plants of indeterminate growth, that is, flowering, fruiting, and vegetative growth occur at the same time (Fischer et al. 2011). Rodríguez-Burruezo et al. (2011) stated that the exploitation of the genetic variation of the Andean Solanaceous fruit trees increases the opportunity to achieve the adaptation of these crops to the subtropical climates.
Plant ecophysiology is understood as the study of the plant behavior in a particular habitat, which helps to recognize their performance in cultivation and, in addition, facilitates management decisions by the producer, but always taking into account that the maximum production of a species, that is, its genetic potential, can only be obtained when the environmental conditions are close to optimal (Pérez & Melgarejo, 2015). Likewise, for the researcher, in-depth knowledge of the physiological responses that originate from the different types of abiotic stress improves the design of methods and mechanisms to increase the tolerance of plants to different stresses (Ngasoh et al. 2019). The ecophysiological factors that affect these crops are mainly altitude, temperature, solar radiation, precipitation, and wind (Fischer & Melgarejo, 2020; Restrepo-Díaz & Sánchez-Reinoso, 2020). And the effect of air pressure that decreases with altitude should not be forgotten because it influences different characteristics of the plants, such as the number of stomata (Fischer & Melgarejo, 2020). Due to the absence of temperature seasons that influence plant physiology in the tropics, these are replaced by the rainy and dry seasons (Fischer & Parra-Coronado, 2020).
No ecophysiological factor acts alone, but these always appear together, that is, ecophysiology is a multidimensional discipline and many trials done under the phytotron conditions with only one or two changing factors are hardly applicable to the reality of the climate in a defined area (Fischer et al. 2016; Restrepo-Díaz & Sánchez-Reinoso, 2020). Likewise, the conditions of one country are only partially applicable to another country or area different from the original one (Fischer & Orduz-Rodríguez, 2012). Recording the physiological and growth responses of plants to environmental factors is not always an easy tusk to achieve in cultivation (Saavedra et al. 2020), for which, in many cases, artificial growth facilities (greenhouses, growth chambers, phytotrons) and sophisticated equipment are employed for the measurement of these ecophysiological variables. Some progress has been made in the recent decades, which was reflected in increasing productivity of fruit trees, however, these are now being re-evaluated due to the impacts of climate change (Restrepo-Díaz & Sánchez-Reinoso, 2020).
The objective of this literature review was to present the important ecophysiological information on four fruit species of the Solanaceae family of the Andes, cape gooseberry, tree tomato, lulo, and sweet cucumber, to illustrate their climatic requirements and the climate effects on their physiology as the bases for their sustainable cultivation and genetic improvement of the crops. On the other hand, and in accordance with Cronin et al. (2008) this literature review can provide the basis for future research projects.
MATERIALS AND METHODS
This document consists of a literature review, for this reason, it was necessary to assess an information in different databases through the Internet, including Science direct, Scopus, Scielo, and Google Academic. The search was made using the keywords (in English and Spanish) such as “fruit cultivation”, “ecophysiology”, “cape gooseberry”, “tree tomato”, “lulo”, “sweet cucumber”, “cold climate fruit growth”, Physalis peruviana, Solanum betaceum, Solanum quitoense, and Solanum muricatum. From these databases, 73 sources were obtained that include web pages (from reliable authors), books, and (mostly) scientific articles from the last 31 years, in English and Spanish, both from national and international scientific journals. Interestingly, the most of these sources were both Colombian journals and authors; this indicates that the largest amount of research on the ecophysiology of the species treated in this review has been carried out in Colombia.
RESULTS AND DISCUSSION
Climate change aspects of the Andes. Marengo et al. (2011) reported that precipitation rates in the tropical Andes will increase by 20 to 25%; additionally, for many fruit crops, such as cape gooseberry, the global warming will have a higher impact on their growth at the low altitudes. The IPCC technical summary (Shukla et al. 2019) clearly indicates that almost all fruits and vegetables, which are key elements for a healthy diet, are among the crops most susceptible to climate change and that their yield and quality will be reduced as warming increases, especially in tropical and subtropical areas.
In their very extensive study on the phenology of fruiting in the American Neotropics, Mendoza et al. (2017) found that the climatic factor that mostly regulates this reproductive phase is rain (73.4%), followed by air temperature (19.3%), solar radiation or photoperiod (3.2%), and only the 1.4% physiological events is attributed to the Niño-Southern Oscillation (ENSO).
Not only in the Andes the fruit production is subject to growing uncertainty in the areas where negative climate changes are expected. The climatic alterations can also be considered to increase fruit production in some areas until they drive a productive expansion (Raza et al. 2020; Fischer & Melgarejo, 2021). Thus, the advantage of the Andean crops, according to the authors of the present review, is that the increase in temperature due to global warming might not affect them as much, because these can be grown at a higher altitude and, thus, find the optimal temperature. Crop physiologists, such as the Brazilian, DaMatta et al. (2010) suggested that global warming will cause crops to grow faster, with small changes in their development, such as flowering and fruiting, depending on the species. They also proposed that C3 crops (most fruit trees) will, possibly, produce larger plants using less water due to the increase in atmospheric CO2, in the event that other stress conditions do not occur. In addition, it should be considered that Ligarreto (2012) classified these Andean crops as having a minimal environmental impact.
Ecophysiological aspects of the tropical altitude. The tropical altitude is suitable for many fruit species, also for some native to other regions of the world, some thriving up to 3,000m a.s.l., taking into account that the frost season should not affect the reproductive growth of the plants (Fischer & Orduz-Rodríguez, 2012). At the same time, the tropics have an advantage for being the regions with thermal uniformity, that is, the monthly average temperatures almost do not change during the year and, on the other hand, high solar radiation favors fruit formation with a thick skin and high antioxidant content, that be a prominent aspect for consumers, but also, in extreme cases, causing a fruit sunburn (Fischer, 2000; Fischer et al. 2016).
Regarding the growth and phenological phases of fruit trees, the temperature, which decreases with altitude (about 0.6°C per each 100m) increases the duration of these phases, that is, the fruit ripening happens later than in the lower areas still suitable for the cultivation (Fischer & Orduz-Rodríguez, 2012). Furthermore, as these authors mention that, with increasing altitude the partial pressure of gases (CO2, O2, N2) and water vapor is reduced as well as the precipitation decreases from 1,300 to 1,500m a.s.l., while the intensity of the visible and infrared UV radiation and wind speed increase.
These fruit trees as well as, in general, the plants of the high Andean regions are of the lower size, possibly due to the influence of ultraviolet (UV) light on the production of auxins (Fischer & Melgarejo, 2014) and/or the effect of UV light on the minor synthesis of gibberellins in the internodes (Buchanan et al. 2015), compared to those that grow at lower elevations. Additionally, the fruit trees grown at the high altitudes have lesser leaf expansion, and the leaves are thicker due to an increase in the number of parenchyma layers and a thicker cuticle to better resist UV light (Fischer & Miranda, 2021).
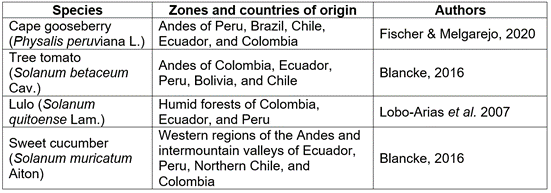
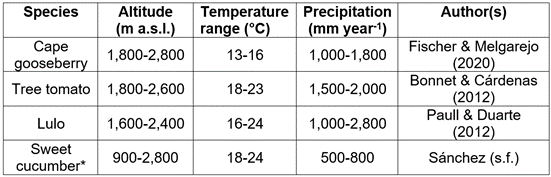
The cape gooseberry (Physalis peruviana L.). As a typical plant of the Andes (Tables 1, 2; Figure 1a) it finds very favorable conditions for growth, production, and quality in Colombia between 1,800 and 2,700m a.s.l. (corresponds to temperatures of 13 to 16°C (Table 2) (Fischer & Melgarejo, 2020), with altitudes between 2,200 and 2,400m a.s.l. being the best conditions for the commercial production, while Blancke (2016) mentioned its general adaptation in the Andes between 1,000 and 3,000m a.s.l. This species is highly adapted to low temperatures, has a base temperature (minimum) for stem development of 6.3°C and only 1.9°C as a minimum temperature for fruit development (Salazar et al. 2008). Its leaves, flowers, calyxes, and young fruits do not resist temperatures below 0°C, while high temperatures (>30°C) cause the abortion of flowers (Fischer & Melgarejo, 2014; Carrillo-Perdomo et al. 2015).
Table 2 Altitudes, temperatures, and precipitation recommended for various Solanaceous fruit crops in the Andean region of Colombia.
* Recommended for the conditions of Peru.

Figure 1 Plants in reproductive stage. a) cape gooseberry; b) tree tomato; c) lulo; d) sweet cucumber fruits.
It is highly adapted to high solar radiation and abrupt changes between the day and night temperatures, due to the calyx that encloses and protects the fruit and the trichome layer that covers the entire green part of the plants, especially the leaves (Ramírez et al. 2013). It is possible that due to the reduced gas pressure with increasing altitude, the plants form a greater number of foliar stomata in the areas of higher altitude (Fischer & Melgarejo, 2020). The solar radiation incident on the calyx and the two adjacent leaves, with which the fruit grows in each node of the reproductive part of the plant, are especially important for fruit filling (Fischer et al. 2015). Some 1,500-2,000h of sunlight per year favor the overall performance, yield and fruit quality (Mora et al. 2006). This crop showed a foliar photosynthetic rate under the conditions of Bogotá (2,600m a.s.l.) equal to 10.545μmol CO2 m-2 s-1 (Fischer & Melgarejo, 2020).
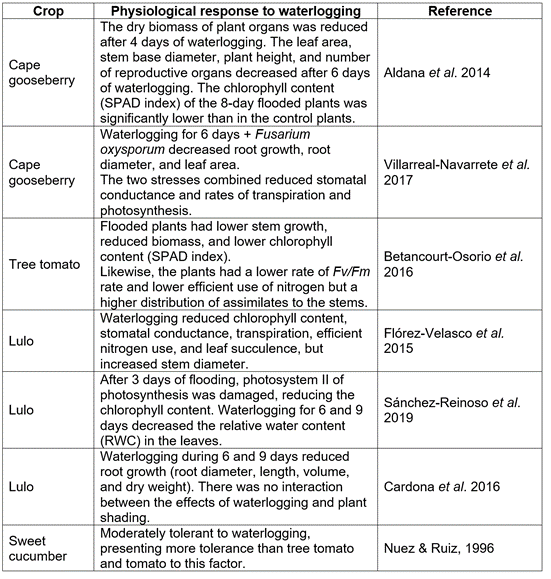
A constant water supply is essential for the indeterminate growth of this plant and a rainfall of 1,000 to 1,800mm year-1 evenly distributed throughout the year is favorable for its performance (Fischer & Melgarejo, 2020). However, the soil moisture level must always be slightly below field capacity because cape gooseberry, in general, does not withstand waterlogging for more than 4 days (Table 3) (Aldana et al. 2014). Prolonged rainy seasons or heavy rains after a dry season can cause fruit cracking, which is the most important physiological disorder in this species and which can be alleviated with optimal fertilization with calcium, boron, potassium, and magnesium (Torres et al. 2016; Garzón-Acosta et al. 2014). Severe water stress, which occurs especially during the El Niño phenomenon, reduces plant growth, resulting in smaller leaves and fruits (Fischer & Melgarejo, 2014). Torres et al. (2004) recorded that water stress, particularly during the first weeks of the reproductive phase, generates smaller fruits and lower productivity in general. Álvarez-Herrera et al. (2019) found that an irrigation coefficient of 1.1 originated higher values of the efficient water use of the Ψleaf and Ψstem, which is the most recommended for a production of large fruits and with less cracking.
Table 3 Physiological response of Cape gooseberry, tree tomato, lulo, and sweet cucumber to waterlogging in the Andes.
For the productive areas in Ecuador, Carrillo-Perdomo et al. (2015) reported altitudes up to 3,300m suitable for cultivation of cape gooseberry, with an optimum temperature of about 18°C, high incident solar radiation, and annual rainfall of 1,000-2,000mm. The cape gooseberry does not resist high wind speed (Carrillo-Perdomo et al. 2015) due to the deformation and breakage of branches and leaves, also due to the fall of the reproductive organs (Fischer & Melgarejo, 2014). This situation that makes it necessary the installation of living barriers against the wind, which also favors the flight of pollinating bees for this species that might no longer fly at wind speeds superior than 10km h-1 (National Research Council, 1989). On the contrary, low winds benefit the maintenance of a stable CO2 concentration in the plant to guarantee the optimal photosynthesis (Fischer & Orduz-Rodríguez, 2012), but in cape gooseberry it is necessary to quantitatively evaluate these aspects.
In general, hailstorms that can occur sporadically in many producing areas, affect these four Solanaceae fruit crops, causing impacts by hail, depending on their diameter, speed, density, and duration, in all green parts, especially in the leaves, but also in flowers, fruits, tender shoots and, in the case of cape gooseberry, in the calyxes, in addition, causing the fall of these organs. However, if the damage was not so serious, the plant can recover and terminate its vegetative and reproductive growth cycles (Ramírez & Kallarackal, 2019; Fischer & Orduz-Rodríguez, 2012). In the case of larger losses and damages that decrease the plant production and predispose these to infections by pathogens, it is recommended to install the crops in areas with little probability of this phenomenon and, in any case, the application of fungicides is advisable, such as the protectants to prevent infection of damaged tissues by pathogens (Torres et al. 2016).
The tree tomato (Solanum betaceum Cav.). In Colombia, the tree tomato or tamarillo (Figure 1b) is found in the wild between 1,200 and 3,000m a.s.l. and produces better from 1,800 to 2,600m a.s.l. (optimal 1,900-2,300m a.s.l.), with temperatures between 13 and 20°C (Bonnet & Cárdenas, 2012a). These authors recommend planting this crop in areas with an annual rainfall between 1,500 and 2,000mm well distributed throughout the year but with a short "summer", to have no damage in the production and fruit quality; these suggest a relative air humidity of around 80% and the installation of a drain on flat sites to avoid waterlogging. Due to the danger of anthracnose (Colletotrichum gloesporioides), Angulo (2003) warned of such high levels of air humidity and recommends sites with air humidity of 60 to 70% for its cultivation, also choosing regions with a direct sunlight of about 1,800 to 2,300h per year.
In the Andean equatorial zone, the tree tomato can be found between 1,500 and 3,000m a.s.l. (Lagos et al. 2011), while, in subtropical regions, it can grow up to the sea level and resist light frosts (Blancke, 2016). It does not resist dry periods that especially affect its flowering, due to a very superficial root system (Carrillo-Perdomo et al. 2015; Ramírez & Kallarackal, 2019) requiring additional irrigation under these conditions (National Research Council, 1989). This species subjected to water stress in the vegetative stage decreases growth rate, total dry weight, transpiration, and stomatal conductance (Clavijo-Sánchez et al. 2015).
For the areas of its cultivation in Ecuador, Carrillo-Perdomo et al. (2015) reported that the tree tomato is tolerant to low temperatures, which must be above 10°C, while the temperatures equal to or lower than -2°C cause damage to the seedlings, branches, and young foliage. It grows well in temperature regimes between 13 and 24°C, with optimum temperatures between 16 and 19°C (Chañag-Miramag et al. 2017). In the case of colder subtropical zones, Carrillo-Perdomo et al. (2015) reported suitable altitudes between 300 and 1,000m a.s.l., likewise indicating that low temperatures promote flowering, while excessively hot temperatures affect all reproductive phases and promote rarely set fruits (National Research Council, 1989), at the same time, these processes require more research. The tree tomato does not withstand strong winds due to its fragile branches, soft leaves, and superficial rooting; therefore, it cannot be grown in heavy, compacted, and flooded soils (Carrillo-Perdomo et al. 2015). However, the wind as an abiotic vector, plays an important role in its pollination (Schotsmans et al. 2011; Ramírez & Kallarackal, 2019).
Lulo (Solanum quitoense Lam.). The lulo or naranjilla (Figure 1c) adapts well to the humid high Andean tropics because it requires an annual precipitation between 1,000 and 2,800mm, with an optimum of about 2,500mm, but also thrives well in drier areas with supplementary irrigation (Paull & Duarte, 2012). As for the temperature range for plant growth, these authors recommended 16 to 24°C (optimal 17-18°C) and an altitude between 1,600 to 2,400m a.s.l., favoring 2,000 to 2,400m a.s.l. for the most acidic type of fruits with thorns S. quitoense f. septentrionale and 1,600-2000m a.s.l. for S. quitoense f. quitoense, without thorns. Bonnet & Cárdenas (2012b) attributed the areas located between 2,000 and 2,200m a.s.l. as the most optimal for its development. Pulido et al. (2008), through the regression calculation method, estimated 9.6°C as the base (minimum) temperature for the node appearance on the lulo main stem, while Cruz et al. (2007) found accumulation of thermal units above 8ºC as a growth threshold for S. quitoense f. septentrionale. For the same accession, at the vegetative phenological stage, the photosynthetic rates from 4 to 8µmol CO2 m-2 s-1 were reported, which were directly related to photosynthetically active radiation in conditions of the low montane humid forest (bh-MB) of the Eastern Antioquia (Colombia) (Medina et al. 2006).
Bonnet & Cárdenas (2012b) placed special emphasis on the high-water requirement of these plants, from 1,800 to 3,000mm, well distributed throughout the year, which means a daily water level of about 4 to 6mm. In addition, the lulo is adapted to shady conditions (Cardona et al. 2016), for which Lobo (2006) characterized it as an understory plant, developing large leaves with different insertion angles that allow better capture of solar radiation that passes through the canopies of trees, however, Paull & Duarte (2012) and Casierra-Posada et al. (2013) stated that it also grows and produces well in full sun. In this regard, Lobo (2006) observed that the lulo at full sun exposure accelerated senescence, shortening the productive period.
To ensure a better protection against UV rays, this species develops trichomes on petals and fruits as well as a high anthocyanin content in the vascular system (Fischer & Orduz-Rodríguez, 2012). Bonnet & Cárdenas (2012b) suggested to cultivate lulo in the dark until recommending a black shading net (25-35% shade), because this plant would not close the stomata, which would further conduct to significant loses of water through transpiration, corresponding to a mean transpiration of 50L d-1 of water per plant in extreme cases. In Ecuador, Revelo et al. (2010) stated that a lulo plantation requires a rainfall between 1,500 and 4,000mm year-1, with an optimum of 2,500mm. However, it is to consider (Tab. 3) that the growth of the lulo suffers from waterlogging after 6 days (Cardona et al. 2016) and the photosynthesis is affected by this condition after 3 days (Sánchez-Reinoso et al. 2019). Foliar applications of nitrogen decreased the detrimental effect of waterlogging in the lulo (Flórez-Velasco et al. 2015).
Ramírez et al. (2018) reported that the lulo plants respond to the photoperiod with respect to flowering. Messinger & Lauerer (2015) found under greenhouse conditions in Germany that, in plants under long day conditions (summer in Germany), the period between the flower bud appearance and fruit set becomes shorter, reducing the flowering period and time until harvest. These authors also observed that under short day conditions (winter) the lulo plants produced more flowers than during long days in summer. Likewise, the National Research Council (1989) mentioned that the lulo, possibly, needs short-day environments for its pollination. Due to all the above, the lulo plants need even more research on ecophysiological issues. Since, after Paull & Duarte (2012), the lulo has good rooting and anchoring it should not be very susceptible to uprooting by strong winds, but its large, soft leaves can be seriously damaged.
The sweet cucumber (Solanum muricatum Aiton). The sweet cucumber or pear melon is not as well-known as the first three fruit species of this review, for which the National Research Council (1989) and Herraiz et al. (2016) described it as an Andean crop “neglected” or “lost”; however, it draws a growing interest in many markets for exotic fruits (Rodríguez-Burruezo et al. 2011). Apart from its greater distribution in the western Andean regions and inter-Andean valleys of Ecuador, Peru, North Chile and Colombia, Mathias & Madeira (2017) affirmed for Brazil that the sweet cucumber has a good climatic adaptation to the South and Southeast regions, being also planted in areas of the Midwest of Brazil at altitudes above 1,000m a.s.l.
The fruit set and yield in this crop are affected by high temperatures (Rodríguez-Burruezo et al. 2011), due to the negative effect of heat on pollen fertility (Ruiz et al. 1996). Thus, Nuez & Ruiz (1996) observed abortion of flower buds when temperatures exceeded 35°C and/or conditions of extreme drought, for which these authors recommended growth areas with temperatures below 25°C to guarantee optimal fruit set, while posterior higher temperatures would no longer affect the plant development. Nuez & Ruiz (1996) estimated about 8 to 10°C as minimum night temperatures for the proper fruit set, while temperatures below 10-12°C can affect the fruit development (Infoagro, 2020). Jana (2019) reported between 380 and 550 degree days (base temperature of 10°C) are necessary for flowering to occur, while 400 to 580 degree days are required for fruit set, and harvest requires only at least 1,000 degree days.
However, there are ecotypes that, despite their low pollen fertility, can give high yields because these have facultative parthenocarpy (Rodríguez-Burruezo et al. 2011). These authors reported that the cultivation of sweet cucumber under Mediterranean greenhouse conditions showed a higher yield in the autumn-winter season than in the spring-summer season, probably because the autumn-winter conditions were more consistent with those suitable for the fruit development. Sweet cucumber is susceptible to frost, with damage depending on the duration and magnitude of these conditions, however, after a short frost of -2°C the plants usually recovered from damage done to leaves and small fruits (Nuez & Ruiz, 1996). Due to its very superficial roots, sweet cucumber is susceptible to drought, however, according to Nuez & Ruiz (1996), it recovers quickly. Blancke (2016) observed in sweet cucumber that the color of flowers depends on the temperature because plants in cooler environments below 20°C develop blue flowers and those grown above 25°C develop white flowers, while Herraiz et al. (2015) reported that high temperatures reduced a content of sugars in the fruits, which acquired an unpleasant taste. The intensity of the "exotic" color of fruits (purple stripes, Figure 1d) depends on solar radiation that increases the accumulation of anthocyanin pigments promoting the purple color of flowers and fruits (Jana, 2019).
In contrast to the first three Solanaceae listed in this review, sweet cucumber has been studied for the effects of an environmental enrichment with CO2 (350, 700, and 1,050ppm) in growth chambers in cv. Xotus (Chen et al. 1999a), which increased foliar and fruit growth as well as the net assimilation rate and relative growth with the air enrichment with CO2 to 750 and 1,050ppm. Also, Chen et al. (1999b) found that the net photosynthetic rate and the photosynthetic water use efficiency increased substantially at CO2 levels of 700 and 1,050ppm as compared to 350ppm. Nuez & Ruiz (1996) reported damage to the foliage by strong winds, especially, if these were combined with low temperatures, while Infoagro (2020) also warned of the danger of very hot and dry winds, for which wind curtains are necessary in areas exposed to this factor.
This literature review indicates that the information on these four crops, which are the most widely planted nightshade fruit trees in the Andes, is not very abundant and only began gaining a structure starting from the 1990s. The publications refer mostly to observations of field crops in certain microclimates and altitudinal ranges, where the species prospered very satisfactorily (Bonnet & Cárdenas, 2012a; 2012b).
Some of these adaptations of crop defenses against high UV light intensities include the abundant trichomes in cape gooseberry and lulo (Ramírez et al. 2013), and the greater synthesis of anthocyanins in tree tomato and lulo (Fischer & Orduz-Rodríguez, 2012). Likewise, these species do not withstand high temperatures during flowering, for which a maximum temperature between 25 and 30ºC is recommended (Fischer & Melgarejo, 2014), which shows their nature of fruit trees originating in the Andean areas (Blancke, 2016).
With low knowledge of ecophysiology in these species (Sánchez-Reinoso et al. 2019), more studies of a physiological, biochemical, and even molecular aspects are required, which would allow for the understanding of the influence of climate on photosynthesis, transpiration, respiration, sink-source relationship, hormonal behavior, among others processes, to make more accurate decisions related to the growing of these species in the most appropriate areas to provide a better expression of their genetic potential (Pérez & Melgarejo, 2015).
Especially, it is important to know these species reaction and adjustments to the climate change, such as the increase in temperature or the presence of extreme events such as drought and heavy rains that generate floods and waterlogging (Shukla et al. 2019). This climatic variability affects the physiology of these solanaceous plants and demands the implementation of different agronomic practices for specific management of the temperature and precipitation effects (Fischer & Miranda, 2021). In addition, the effects of raising CO2 concentrations on the growth, physiology, production, and quality of these Solanaceae should be addressed, in order to improve their adaptation to this new ecophysiological situation and to re-evaluate the results of the previous studies (Restrepo-Díaz & Sánchez-Reinoso, 2020).