INTRODUCTION
The Bombus genus (Hymenoptera: Apidae: Bombini) consists of nearly 250 species worldwide (Williams et al. 2008) and is recognized by its potential as a pollinator in certain crops, especially solanaceous and some ericaceous plants (Delaplane & Mayer, 2000; Goulson, 2010; Abrol, 2012). The bees of this genus build underground or ground-level nests using hollow cavities made by other organisms or make domes using branches and leaves (Cameron et al. 1999; Michener, 2007; Goulson, 2010). Worldwide, there is a great concern regarding the health of bumblebee and other pollinator populations, since a food shortage caused by the lack of pollinators could compromise human wellbeing (Potts et al. 2010; Bommarco et al. 2013).
Bumblebee populations can be positively or negatively impacted by other organisms. These organisms have been primarily recorded from nests, foragers, workers, and queens (González et al. 2004; Goulson, 2010). The organisms associated with bumblebees and their nests could interact with them in different ways, being parasites, predators, commensals, detritivores or scavengers (Goulson, 2010). Arthropods such as beetles, flies and mites have been reported inside bumblebee nests (Alford, 1978; Goulson, 2010); but worldwide their geographical distributions and function are poorly understood.
Bumblebees have a great diversity of natural enemies with many records made mainly in Europe, New Zealand, North and South America (Husband & Brown, 1976; Donovan & Wier, 1978; Prys-Jones & Corbet, 1991; Goulson, 2010; Maggi et al. 2011; Gamboa et al. 2015; Revainera et al. 2014; 2019; 2020; Plischuk et al. 2020). Even though natural enemies of bumblebees have been studied for many years, it was only after the development of artificial rearing, mass production and international commercialization between 1960 and 1980 (Velthuis & Van Doorn, 2006), that the research on bumblebee pests, parasites and pathogens received great attention by sanitary agencies and conservationist organizations. These institutions aim to reduce the risks posed by the introduction of invasive organisms, such as foreign bumblebee species used for pollination in greenhouses and orchard crops (Prys-Jones & Corbet, 1991; Goka et al. 2001; Morales, 2007; Williams & Osborne, 2009; Goulson 2010; Hatfield et al. 2012).
Natural enemies of bumblebees have mostly been reported attacking bumblebee nests and adults. Vertebrates such as badgers, skunks, foxes, moles, weasels, shrews, voles, minks and mice are the most common natural enemies that have been reported attacking nests (Prys-Jones & Corbet, 1991; Goulson, 2010). Records of natural enemies attacking adults include predators such as birds, spiders, robber flies (Diptera: Asilidae) and the wasp Philanthus bicinctus (Hymenoptera: Crabronidae).
Husband & Brown (1976) reported more than 50 species of arthropods, including insects and mites, associated with bumblebee nests in USA and Europe. The way these arthropods impact bumblebee colonies is poorly understood. They could play a role recycling nest debris and bumblebee corpses that accumulate inside the nest, as bumblebees themselves do not have the cleaning behavior of other social insects like the honeybees that remove remains from their nest. Mites establish strong associations with bumblebee nests and adults, but little is known about their role and authors only report them as either being phoretic or parasites (Maggi et al. 2011; Klimov et al. 2016). Other group of organisms associated with bumblebee colonies are nematodes, which have been shown to impair the queen’s reproduction (Prys-Jones & Corbet, 1991).
The main concern about organisms associated with bumblebees, in both natural and artificial rearing settings, is the introduction of invasive species, which can spill over into their habitat competing for resources like nesting sites and food, impacting native bumblebee species and spreading pathogens such as fungi, protozoa, nematodes, and viruses. Invasive species also include arthropods, which have been found attacking bumblebee colonies under laboratory and field conditions. Examples include the bumblebee wax moth, Aphomia sociella (L.) (Lepidoptera: Pyralidae), which was first reported in Europe and introduced to USA and Canada (BugGuide, 2020), the Indian meal moth Plodia interpunctella (Hübner, 1813) and the greater wax moth, Galleria mellonella (L.) (Lepidoptera: Pyralidae), which were found in rearing facilities (Kwon et al. 2003; Williams, 1997) and the small hive beetle Aethina tumida Murray (Coleoptera: Nitidulidae) that was found in the field (Spiewok & Neumann, 2006; Hoffmann et al. 2008).
In Colombia, Antherophagus (Coleoptera: Cryptophagidae) beetles have been reported in association with nests of Bombus pauloensis Friese 1913 (formerly recognized as B. atratus Franklin 1913; see Moure & Melo, 2012) (Roubik & Wheeler, 1982; González et al. 2004). This species is probably the most common representative of the genus Bombus in Colombia given its distribution and abundance in entomological collections (Liévano et al. 1991; Téllez-Farfán & Posada-Flórez, 2013). In this work, we report the organisms associated with a B. pauloensis nest found in a field in the Sabana of Bogotá, Colombia.
MATERIALS AND METHODS
We collected and relocated a B. pauloensis nest that was found in a field, on the slope of a deep drainage ditch on the Thomas van der Hammen Natural Reserve in the Sabana of Bogotá (Colombia) (4° 48’ 10.36” N; 74° 03’ 03.45” W) (Figure 1a). Relocation of the nest was necessary because landscape workers were afraid of being stung and set fire to the nest to eliminate the colony. The nest was transferred to a wooden box, placed inside a styrofoam box for temperature insulation and then relocated close to a honeybee apiary that was also located in the Thomas van der Hammen Reserve. The relocation process was done at 6:00am, when all the bumblebees were still inside the nest, making it easier to collect all adults and cells with brood and food. The colony contained approximately 20 queens, 600 females and 200 males, which indicated that it was in the reproductive phase (Prys-Jones & Corbet, 1991). Inside the nest, we found several layers of cells and substantial debris, including plastic bags, nylon cords and leaves of dry grass, suggesting these bees probably took over a mouse nest and used those materials to insulate the nest.
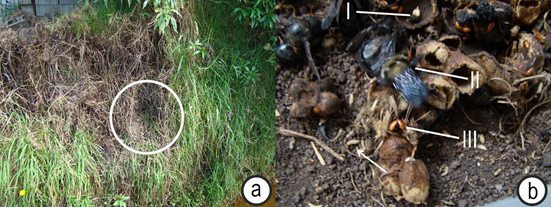
Figure 1 a. Location of the bumblebee nest that was relocated; circle shows the nest entrance; b. B. pauloensis nest showing fly larvae (I), bumblebees (II), and beetles (III).
While digging out the Bombus colony, we observed the presence of beetles, flies, and mites inside the nest (Figure 1b). Samples of those organisms were collected and stored in 95% ethanol and transfer to the laboratory for further observation, identification, and photo documentation. Morphology description, DNA analysis and expert consultation were used to identify the associated organisms found (Peterson, 1957; Arnett et al. 2002; Nelson et al. 2007; Majka & Langor, 2010; Klimov et al. 2016).
The fly species was identified morphologically (Peterson, 1957) and molecularly using genomic DNA, which was extracted from half the body of an adult specimen using the DNeasy tissue kit (Qiagen Inc., Valencia, CA) according to manufacturer’s instructions. One µL of extracted DNA was used as a template to amplify the Citochrome C Oxidase (COI) barcoding region using primers LCO1490-L and HCO2198-L (Nelson et al. 2007). A polymerase chain reaction (PCR) was carried out in a final volume of 50µL, using the EmeraldAmp® GT-PCR Master Mix (Takara Bio Inc., Mountain View, CA) following PCR primer conditions described by Nelson et al. (2007). The PCR product was purified and sequenced by Retrogen, Inc. (San Diego, CA) using respective primer pairs. Forward and reverse sequence files were imported into Sequencher 5.0 (GeneCodes, Ann Arbor, MI) and assembled into one contig sequence using default parameters. The sequence was then compared against the NCBI nucleotide BLAST (nr/nt) database (http://blast.ncbi.nlm.nih.gov) using the megablast algorithm. The barcode sequence obtained in this study was deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/index.html) under accession number KF751463.
For taxonomic identification of mites, samples were sent to Dr. Barry O’ Connor, from the University of Michigan. The identification of beetles was based on taxonomic keys and descriptions provided by Majka & Langor (2010) and Arnett et al. (2002).
RESULTS AND DISCUSSION
One hundred thirty Antherophagus sp. beetle adults, larvae and pupae were found in the B. pauloensis nest (Figure 2a-c). Adult beetles were actively crawling, but the most remarkable observation was that they were attached to B. pauloensis adult legs and antenna and did not dislodge easily when the bumblebees moved away, or when placed in alcohol or into a freezer (Figure 2d). These organisms were previously noted by Roubik & Wheeler (1982) and González et al. (2004), from a B. pauloensis nest in Colombia. The latter authors observed Antherophagus individuals in old combs and debris, and in the upper nest areas looking for workers when the colony was nearly gone. This genus has been reported in other bumblebee and stingless bee (Apidae: Meliponini) nests and has been described as having both phoretic and scavenger behaviors (Wheeler, 1919; Schwartz, 1948; Alford, 1978; Thorp et al. 1983; Goulson, 2010). Alford (1978) and Goulson (2010) reported A. nigricornis associated with bumblebee nests. Antherophagus, also known as flower beetles or silken fungus beetles were found in a nearby honeybee hive alongside pollen and wax on a greased sticky board placed on the bottom of the hive to trap Varroa destructor (Acari: Varroidae) mites. Flower beetles may have been brought to the honeybee hive on bees that were observed robbing the rescued B. pauloensis nest.

Figure 2 Antherophagus beetles found in the B. pauloensis nest. a. Adults; b. Larvae and pupae; c. Ventral view of adult beetle; d. Antherophagus adult beetles attached to the bumblebee legs.
Other arthropods present in the B. pauloensis nest were wireworm (Coleoptera: Elateridae) larvae and adults, morphologically identified to family level. Elateridae larvae are known pests of several crops and can also prey upon other insects (Arnett et al. 2002). Their presence in the nest indicates that they may prey on B. pauloensis brood, on their associated commensals, or are scavengers.
Adults, pupae, and larvae of Fannia canicularis (Diptera: Fanniidae) were also found in the B. pauloensis nest (Figure 3a-c). The 661 bp COI fragment sequence obtained from the specimen showed >91% identity to other COI sequences of the same species (i.e., KY511165, KC617820, and MF511733). Adult flies were not actively flying but rather moving around the cells when the nest was exposed and open. Some specimens lacked wings. Adults and larvae of F. canicularis have been reported in bumblebee nests by Alford (1978) and Goulson (2010). Thorp et al. (1983) reported the genus Fannia as scavengers of bumblebee nests. The Fanniidae family has been reported in other hymenopteran nests such as yellow jacket wasps apparently feeding on debris (Husband & Brown, 1976; Grzywacz et al. 2012). MacDonald et al. (1980) and Grzywacz et al. (2012) mentioned that the function of Fanniidae on Hymenoptera nests seem to be the recycling of debris that accumulate at the bottom of the nest after becoming wet and moldy, when the colonies are declining at the end of the season in temperate regions.
Mites were also observed crawling actively over the cells and debris of the B. pauloensis nest. No mites were observed riding on B. pauloensis adults, but they were rather spread throughout the nest. Mites were taxonomically identified by Dr. Barry O’ Connor as probable Pneumolaelaps sp. (Mesostigmata: Laelapidae) and Parasitellus sp. (Mesostigmata: Parasitidae). Species of these mites have been reported in Argentina, Uruguay, and Bolivia associated to B. paulensis workers caught with nets in the field or found on bumblebee specimens preserved in entomological collections (Maggi et al. 2011; Revainera et al. 2014; 2019; 2020; Plischuk et al. 2020).
Mites of the genus Pneumolaelaps are commonly associated with bumblebees (Hunter & Husband, 1973; Royce & Krantz, 1989) and have been reported with a worldwide distribution (Hunter & Husband, 1973; Royce & Krantz, 1989; Goulson, 2010; Klimov et al. 2016). In South America, Maggi et al. (2011) reported seven species of mites, including P. longanalis and P. longipilus, that were associated as phoretic of B. paulensis. This makes B. pauloensis the studied bumblebee species with the highest diversity of mites reported in South America (Eguaras et al. 1997; Maggi et al. 2011; Revainera et al. 2014, 2019; 2020; Plischuk et al. 2020). Goulson (2010) and Klimov et al. (2016) also reported Pneumolaelaps mites as phoretic with some preferences for Bombus queens, while Royce & Krantz (1989) proved P. longanalis feed on fresh pollen with nectar that bees carry to the nest. However, the consequences of this type of parasitism or commensalism were not evaluated or discussed by the authors.
Goulson (2010), Maggi et al. (2011) and Klimov et al. (2016) also reported Parasitellus as a scavenger mite with obligatory association to bumblebees, present in rearing facilities and with a worldwide distribution. It is important to highlight that the Parasitellus mite collected and identified in this study probably belongs to an undescribed species, which indicates the need to further studying this fauna in Colombia.
From this research the existence of a variety of organisms associated with the B. pauloensis nest found in the Sabana of Bogota (Colombia), which were not causing any negative impact on the bumblebees, was concluded. However, it is necessary to determine the roles and impacts of the associated organisms found under natural nesting conditions to improve the bumblebee’s conservation.
















