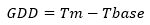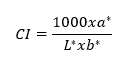INTRODUCTION
The peach (Prunus persica [L] Batsch) belongs to the Rosaceae family (Liu et al. 2020) and is highly desired for its pleasant taste, aroma, and nutritional characteristics (Africano-Pérez et al. 2016; Rodriguez et al. 2019), used for fresh consumption and industrial processes (Mariño-González et al. 2019). By 2018, Colombia had 2,501.62 cultivated ha that produced 34,738.09 t (MADR, 2018). In the country, it is considered one of the most important fruit trees in the high tropics because it has comparative advantages over those grown in temperate areas (Pinzón et al. 2014). These advantages include the agroecological offering that, with the use of early, late and intermediate varieties, can provide year-round production for fresh consumption and agroindustrial uses (Castro & Puentes, 2012).
In Boyacá, Colombia, different varieties are grown, such as Dorado and Rubidoux. The Dorado variety is grown between 2.200 and 2.700 meters above sea level and has a low requirement for cold hours and rapid fruit development, making it a highly appreciated variety in Colombia (Miranda & Carranza, 2013). The Rubidoux variety is adapted to areas between 2.400 and 2.800 meters above sea level, with a requirement of 500-700 cold hours.
The fruit growth follows a double sigmoid pattern (Rodriguez et al. 2019), which begins with a rapid growth phase as the result of cell division and elongation. Afterward, important physiological and anatomical changes are evident, with a decrease in the growth rate of the pericarp, lignification, increased endocarp weight, and partial or total seed formation. The accelerated growth of the pericarp is seen with an increased number and volume of cells and intercellular spaces. In the last stage, the fruit reaches its final size, and ripening begins (Rodriguez et al. 2019). However, the development pattern may vary in the many peach cultivars, which do not present the same phenological cycle in the early, intermediate, and late-cycle materials (Dela Bruna, 2007; Bonghi et al. 2011). During fruit development, there are different important physicochemical and phenological parameters whose behavior is specific for each cultivar. These parameters are useful for understanding the physiology and integral process of fruit development. In addition, they are indicative of the quality and can be used as maturity indices to determine optimal harvest and other important crop tasks. Commonly, soluble sugars increase, total acidity decreases, firmness is lost and color changes occur. These changes were observed in the Jarillo cv, cultivated in three zones in Colombia (Quevedo et al. 2018).
Studies have been carried out for physicochemical and phenological characterizations during the development of peach fruits; however, most have been carried out in temperate zones and do not represent the growth and development conditions of fruits in tropical and subtropical zones where ecophysiological conditions are different (Fischer et al. 2011). Therefore, the objective of this study was to evaluate fruit development with the determination of physicochemical changes and phenology as a function of thermal time in the 'Dorado' and 'Rubidoux' varieties, grown in Colombian high tropics.
MATERIAL AND METHODS
The field phase was carried out on commercial farms in the municipalities of Tuta and Soracá, Boyacá-Colombia. The farms are in the high tropics with altitudes over 2,500 meters above sea level, with an average temperature of 13 to 14 °C and bimodal precipitation that averages 700 to 1000 mm. The laboratory phase was carried out in the Laboratorio de Calidad y Poscosecha de Productos Agrícolas in the Facultad de Ciencias Agrarias at the Universidad Nacional, Bogotá.
15 sampling units were used to collect data with a total of 51 trees selected at random for each cultivar. Each unit had three trees in the full productive stage (7 years of sowing), planted at distances of 3 m between trees and 2.5 m between rows. Each tree had 100 marked flowers, with mixed branches with one year in full bloom. Although only 25 fruits per tree were collected for the analyses, overestimated sampling was used to ensure the total number of fruits needed for this study. The accumulation of Growing Degree Days (GDD) was determined by the following equation:
Where, Tm is the mean daily air temperature, calculated by taking the daily temperature every 30 min with a Humidity and Temperature Datalogger RHT20 (Extech™). The Tbase used the base temperature, which for this study was 4,5 ºC according to Chaar & Astorga (2012).
Starting at day 30 after full flowering (daff), when different measurements could be taken on the fruits, and until harvest, samples were taken every 15 days until each cultivar reached physiological maturity. The size of the repetition varied according to the variable, 10 repetitions for the physical variables, each with one fruit, and 6 repetitions for the determination of the chemical variables. The fruit phenology was established with a photographic record and description of the physicochemical changes concerning the GDD from the formation of the flower bud to harvest.
For the fruit firmness (N), the equatorial zone was drilled with a digital texturometer (Lloyd LS1 brand, Bognor Regis, UK), a 1 KN load cell, a 3 mm cylindrical punch, and Nexygen plus. The titratable acidity (TA % of malic acid) was obtained with acid-base titration according to Africano-Pérez et al. (2016). The soluble solids (SS) were quantified using a Hanna brand digital refractometer with a range of 0 to 85 % with a precision of 0.1 °Brix. The epidermis and pulp color index (CI) were calculated with the CIELab system parameters: L*, a*, and b*, for which three readings were taken in the equatorial zone of 8 fruits with a CR 410 digital colorimeter (Konica Minolta®, Hong Kong). The values were plugged into the following formula:
To determine the fruit respiration (mg CO2 kg-1 h-1), a CO2 sensor was used (Vernier, Software & Technology, Beaverton, OR, USA) with LabQuest according to Mariño-González et al. (2019). The readings were taken every 5 s for 5 min, and approximately 600 g of fruit were used per repetition.
All variables were subjected to descriptive analysis to estimate the mean and standard error in each sampling point, and the coefficient of determination (R2) determined the most appropriate statistical model. the models were generated through the lm function of the software R version 3.5.3.
RESULTS AND DISCUSSION
Phenology and growth. The varieties studied present a differentiated behavior in the development of the fruit, the Dorado variety presented full flowering with fruit set one month later, when the fruit began the first phase, which is characterized by active cell division. This phase lasted 45 d, with 413.5 GDD. A second phase with cell expansion and simultaneous lignification of the endocarp lasted 60 d and accumulated 565.6 GDD. The final phase of fruit ripening lasted 18 d and accumulated 102.7 GDD. This variety accumulated a total of 1081.8 GDD over 153 d after full flowering (daff) (Figure 1).
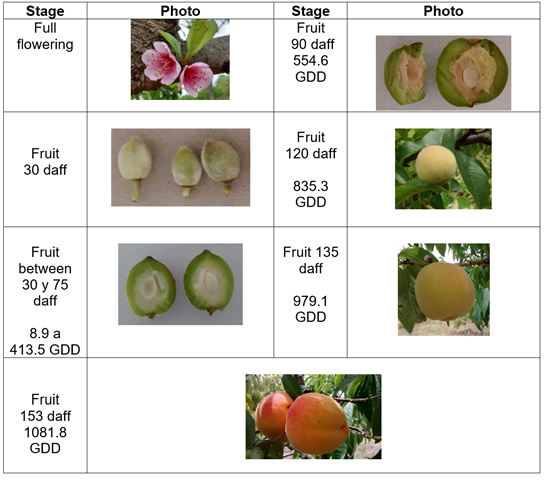
Figure 1 Growth and development of peach fruit cv ‘Dorado’ under conditions of high Colombian tropics. Stages: a) Full flowering; b) fruit 30 daff; c) fruit between 30 and 75 daff, 8.9 to 413.5 GDD; d) fruit 90, daff 554.6 GDD; e) fruit 120 daff, 835.3 GDD; f) fruit 135 daff, 979.1 GDD; g) fruit 153 daff, 1081.8 GDD.
The Rubidoux variety showed full flowering with fruit set one month later, when the first phase began, lasting 60 d and accumulating 563.8 GDD, with little gain for the diameter and mass. Cell division processes predominated up to the second phase, which lasted 90 d and accumulated 734.5 GDD. This phase had an endocarp lignification process that stopped growth, followed by an accelerated gain in mass and size.
The endocarp lignification phase has been described as a prolongation of phase I, characterized by a progressive reduction in the mitotic rate and differentiation of endocarp cells that considerably increase their lignin content (Dardick et al. 2010). The final phase lasted 25 days and accumulated 368.6 GDD. This phase had the greatest amount of physical and chemical changes as maturation occurred. This variety accumulated a total of 1667.1 GDD over 205 daff (Figure 2).
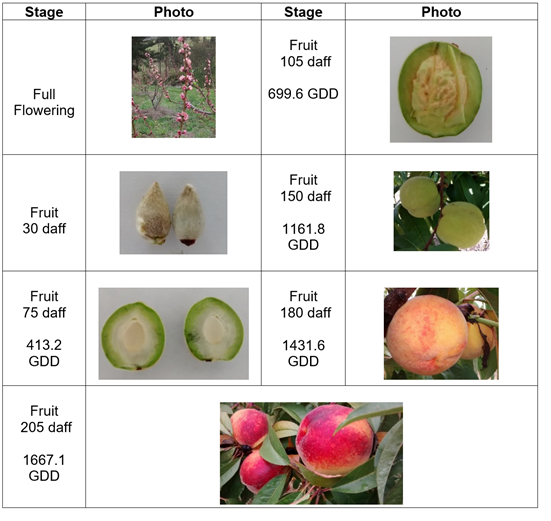
Figure 2 Growth and development of peach fruit cv ‘Rubidoux’ under conditions of high Colombian tropics. Photos: E. Pinzón-Sandoval.
Reports have stated that the accumulated growth of peach fruits follows a double sigmoid model (Rodriguez et al. 2019) but is linked to the cultivar cycle, with some conforming to simple sigmoid models (Dela Bruna, 2007). According to growth and development, peach fruits have three phases. Phase I is characterized by rapid growth as the result of cell division, and phase II has a temporary decrease in pulp mass gain, increased endocarp mass, lignification of the endocarp, with partial or total seed formation. Phase III includes typical physical and biochemical changes for the ripening process, such as an increase in the production of ethylene and volatile compounds, color changes, increase in respiratory rate, decrease in firmness, and chemical transformations that affect sugars, organic acids, proteins, phenolic compounds, pigments, and pectins. During the ripening phase, specific flavors and odors, along with increased sugars and decreased acidity, are notable (Martinez-González et al. 2017). These phases were evidenced in the evaluated cultivars.
Physicochemical changes. The firmness was adjusted to following polynomial models, y = -6E-05x2 + 0.0542x + 27.387 to Dorado (R2 0.94) and y = 3E-08x3 - 0.0001x2 + 0.0959x + 24.057 to Rubidoux (R2 0.92), with a similar behavior in the two evaluated varieties. The Dorado variety presented an initial firmness of 26.72 ± 1.13 N, which increased until reaching a maximum value of 39.40 ± 0.71 N at 413.5 GDD (75 daff). Afterward, it decreased until reaching a value of 11.6 ± 0.78 N at 1081.8 GDD (153 daff) (Figure 3A). The Rubidoux variety had an initial value of 20.17 ± 0.27 N, which increased until reaching a maximum value of 49.03 ± 1.07 N at 285.02 GDD (60 daff). Afterward, it decreased and obtained a value of 9.19 ± 0.40 N at 1667.1 GDD (205 daff) (Figure 3C).
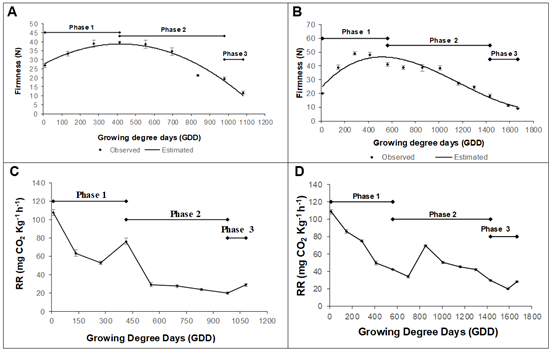
Figure 3 Firmness during the growth and development of the peach fruit: A) cv ‘Dorado’; B) cv ‘Rubidoux’. Respiration rate development of the peach fruit: C) cv ‘Dorado’; D) cv ‘Rubidoux’. Vertical bars at each sampling point indicate the standard error (n=10).
Firmness is the best indicator at a practical level for fruit ripening in different stages. It can determine the optimal levels of consumption, transport, and handling. It is also one of the main quality attributes and often limits postharvest shelf-life (Africano-Pérez et al. 2016). It has been shown that the state of maturity in peaches is highly correlated with pulp firmness (Matteoli et al. 2015). The increase in firmness in the first stages of development in the two peach cultivars, according to some authors, may be associated with the synthesis of protopectin and the accumulation of reserve sugars, such as starch (Lo Bianco & Rieger, 2002; Cirilli et al. 2016).
The loss of firmness in phases 2 and 3 may be due to a series of genetically programmed events, characterized by biochemical and physiological processes. According to Payasi et al. (2009), this process is complex and involves three subsequent steps: 1) relaxation of the cell wall by expansins; 2) depolymerization of hemicelluloses; and 3) depolymerization of polyuronides by polygalacturonase or other hydrolytic enzymes. In climacteric fruits, the loss of interconnectedness in pectins and hemicelluloses results from the effects of solubilization and enzymatic depolymerization and leads to interconversion of highly interconnected polysaccharides to structures with low fibrillar association (Prasanna et al. 2003).
The respiratory rate in the Dorado variety had an initial value of 107.6 ± 3.27 mg CO2 Kg-1 h-1, with a decrease until reaching a value of 53.30 ± 2.01 mg CO2 Kg-1 h-1 at 273.5 GDD (60 daff), followed by an increase until reaching a value of 76.62 ± 3.33 mg CO2 Kg-1 h-1 at 413.5 GDD (75 daff), and finally decreasing constantly to a value of 20.26 ± 1.47 mg CO2 Kg-1 h-1 at 979.1 GDD (135 daff) (Figure 3B). The variety Rubidoux had an initial value of 108.74 ± 2.75 mg CO2 Kg-1 h-1, which decreased to a value of 33.92 ± 1.5 mg CO2 Kg-1 h-1 at 699.6 GDD (105 daff), followed by an accelerated but momentary increase at 855.6 GDD (120 daff), from which it decreased continuously to a value of 28.1 ± 0.1 mg CO2 Kg-1 h-1 at 1586.8 GDD (195 daff) (Figure 3D).
Respiration may be related to the synthesis of new tissue (growth respiration) and structural maintenance (maintenance respiration) through the turnover of ATP and NADH2, which are necessary for plant growth (Galho et al. 2007) and, in this case, for fruit development in the two peach cultivars, especially in phase 1, where the respiratory rate must be high to meet the energy demands of cell division in this stage.
It has been shown at a physiological level that the expression of lignin and flavonoid pathway genes is associated with development processes in peaches. Here, growth is described by double sigmoid curves (Rodriguez et al. 2019), with a growth plateau that coincides with endocarp hardening, commonly known as bone. According to Dardick et al. (2010), the slowdown in fruit expansion could be attributed to important energy resources obtained through respiration processes being used for lignification and hardening of the endocarp because expression of the lignin gene is induced to extremely high levels immediately before the growth slowdown. However, these energy resources appear to be carefully divided to allow the accumulation of flavonoids before the hardening of the endosperm depletes the energy and metabolic resources needed for the other phases of fruit development.
In the studied cultivars, there was an unusual increase in respiration in phase II of fruit growth, which might be due to the fruit lignification process, where growth respiration increases notably to obtain necessary compounds for the formation of this tissue. Despite being unusual, this increase has been reported for peaches grown under subtropical conditions (Silva et al. 2013). A similar result was also found by Hernández & Hernández (2012), who found an increase in the respiratory rate in phase II of growth that was associated with the formation of seeds when studying the Amazonian copoazu fruit.
The epidermis and pulp color index was carried out from 75 daff in the two varieties because the high number of pubescences and the fruit size prevented the correct measurement of this variable up to this time. Epidermis color index of the Dorado variety was fitted to model y = 3E-05x2 - 0.0277x + 1.9834 (R2 0.91), presented a value initial of -5.74 ± 0.31, while the pulp (y = 3E-05x2 - 0,0343x + 2,084) (R2 0.96) was -6.67 ± 0.11, which continuously increased until reaching 2. 30 ± 0.35 and 2.64 ± 0.13, respectively (Figure 4A and B). The variety Rubidoux had an initial epidermis color index of -7.38 ± 0.08, and the pulp had -7.23 ± 0.10, which continuously increased until reaching values at harvest of 7.92 ± 1.27 and 3.21 ± 0.18, respectively (Figure 4C and D), the models to epidermis and pulp were y = 3E-08x3 - 8E-05x2 + 0,0756x - 26.906 (R2 0.96), and y = 8E-06x2 - 0.0082x - 4.7595 (R2 0.96) respectively.
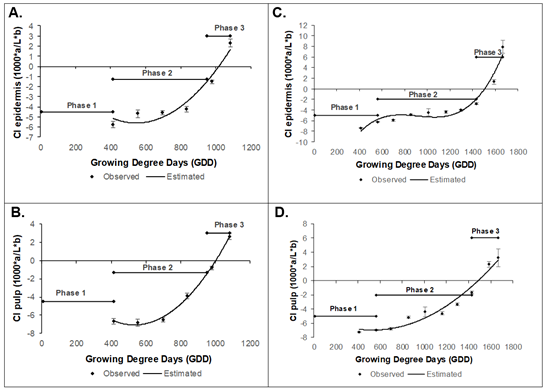
Figure 4 Color Index during the growth and development of the peach fruit cv ‘Dorado. A) Epidermis; B) Pulp and cv ‘Rubidoux’; C) Epidermis; D) Pulp. Vertical bars at each sampling point indicate the standard error (n=10).
Previous results have indicated that both the Dorado and Rubidoux varieties have green fruits, epidermis, and pulp, in early stages of fruit growth, with synchronous epidermis-pulp changes to yellow fruits in the Dorado variety and to intense yellow fruits in the Rubidoux variety, which, according to Pinzon et al. (2014), are characteristic colors for these cultivars.
Color is a fundamental physical property of agricultural products because of its high correlation with other physical, chemical, and sensory indicators of quality in fruits and vegetables. Pigments make fruits attractive and commonly accumulate in the cuticle during the ripening process although many climacteric fruits also accumulate pigments in pulp tissue during ripening (Bouzayen et al. 2010), as seen in figures 1 and 2 for the two peach cultivars. According to Slaughter et al. (2013), the color change is caused by the enzymatic degradation of chlorophylls, synthesis of carotenoids and anthocyanins, and variation in the cell pH. Color change has also been related to an increase in ethylene since it triggers chlorophyll degradation reactions and stimulates coloring in berries by regulating genes involved in anthocyanin biosynthesis (Bouzayen et al. 2010). The results indicated that the color index in cultivars 'Dorado' and 'Rubidoux' is a very reliable parameter for establishing harvest tasks, with synchronized external color (epidermis) and internal color (pulp) under the studied conditions.
Soluble solids (SS) were adjusted to second (y = 3E-06x2 + 0,0018x + 9,5623, R2 0.93) and third order (y = 1E-09x3 - 3E-06x2 + 0,0043x + 8,9633, R2 0.87) polynomial models for Dorado and Rubidoux, respectively. The Dorado variety obtained an initial value of 9.1 ± 0.63 °Brix, until reaching a maximum value of 15.9 ± 0.9 °Brix (1081.8GDD or 153 daff) (Figure 5A). The Rubidoux variety presented a value of 9.0 ± 0.17 °Brix at 9.6GDD (30 dds), with a continuous increase until reaching a maximum value of 15.5 ± 0.3 °Brix at 1667.1GDD (205 daff) (Figure 5B).
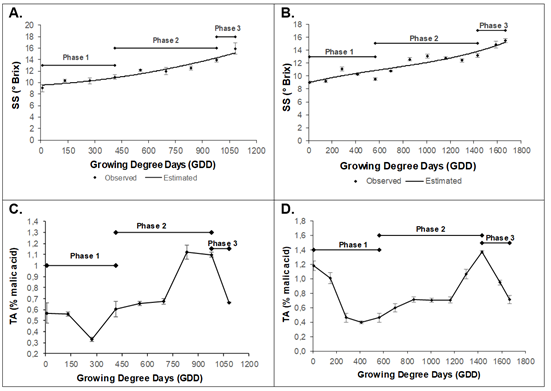
Figure 5 Behavior of Soluble Solids (SS) in peach fruit of A) cv ‘Dorado’; B) cv ‘Rubidoux’. Behavior of Titratable acidity (TA) in peach fruit of C) cv ‘Dorado’; D) cv ‘Rubidoux’. Vertical bars at each sampling point indicate the standard error (n=6).
The peach accumulates different types of soluble sugars and sugar alcohols, mainly sucrose, glucose, fructose, and sorbitol (Cirilli et al. 2016). Sucrose is the predominant sugar in the peach mesocarp at maturity, representing approximately 40 to 85 % of the total sugar content, followed by Glucose and Fructose (in variable proportions) (Brooks et al. 1993). These sugars were possibly in the evaluated cultivars. Starch accumulates in the early stages of fruit development and is then rapidly metabolized, becoming almost imperceptible in the maturity or the final phase (Lo Bianco & Rieger, 2002). Hexoses are the most abundant sugars in immature peach fruits until the phases of rapid growth or cell elongation begin when sucrose becomes the predominant sugar. This behavior may also occur in the Dorado and Rubidoux varieties. While the accumulation pattern of glucose and fructose is generally characterized by variations throughout fruit development, the content is slightly higher in the early stages and progressively declines until maturity (Desnoues et al. 2014).
For the titratable acidity (TA), the variety Dorado presented an initial concentration of 0.56 ± 0.09 % at 8.9 GDD (30 daff), with a decrease up to 273.5 GDD (60 daff). Afterward, there was a continuous increase up to 835.3 GDD (120 daff), reaching a value of 1.12 ± 0.06 %, followed by a drastic decrease until harvest, with a final value of 0.66 ± 0.003 % at 1081.8 GDD (153 daff) (Figure 5C). The variety Rubidoux obtained an initial TA of 1.18 ± 0.06 %, which decreased up to 413.2 GDD (75 daff). Afterward, the TA increased until a maximum value of 1.38 ± 0.01 % at 1431.6 GDD (180 daff). Then, the TA began to decrease until a value of 0.71 ± 0.05 % at 1667.1 GDD (205 daff), when the fruit reached physiological maturity (Figure 5D).
The decrease in acidity observed in the first phase in the two peach cultivars (Figure 5) may have been associated with the consumption of organic acids as a result of the high respiratory rates in this stage, as observed by Quevedo et al. (2018) in variety Jarillo. However, some reports indicate the opposite, where fruits accumulate organic acids in the early stages of development (Cepeda M. et al. 2021). The increase found in phase 2 is uncommon for this species although it has been found in some plums with increased malate that remains constant during ripening and only decreases in a very advanced stage of this process (Famiani et al. 2012). This result is consistent with the findings of this study.
Most of the organic acids present in the pulp of fruits are not imported; they are synthesized in the pulp from sugars (Etienne et al. 2013). They accumulate in the pulp of many types of fruits in certain stages of development (Famiani et al. 2015). The peach may have important organic acids such as oxalic acid, quinic acid, shikimic acid, citric acid, fumaric acid, and malic acid. The latter is the predominant acid (Le Dantec et al. 2010; Famiani et al. 2015). However, TA, a general measure of acids in fruits, can vary between cultivars, as evidenced in our study.
The results allow concluding that the behavior of several physicochemical parameters was elucidated for Dorado and Rubidoux varieties cultivated under high tropic conditions. The fruit development cycle in Rubidoux is greater than in Dorado. The firmness, soluble solids, and color index showed a typical behavior for this species in the two cultivars. The acidity had a marked increase during phase 2 of development but decreased during maturation. The respiratory rate decreased between phase 1 and 3 but had an atypical increase in phase 2 in the two varieties.