INTRODUCTION
Colletotrichum is a genus of the order Glomerellales, family Glomerellaceae that has high diversity and is grouped into several species complexes or clades (Jayawardena et al. 2021; Liu et al. 2022; Talhinhas & Baroncelli, 2021). This genus comprises major plant pathogens that cause Anthracnose diseases in many crops worldwide (Zakaria, 2021; Gañán et al. 2015; Ismail et al. 2015; Sharma & Kulshrestha, 2015; Honger et al. 2014; Liu et al. 2014). Colletotrichum species present different lifestyles, from non-pathogenic endophyte to necrotrophic pathogen; as a causal agent of diseases, it has been considered a hemibiotrophic microorganism (Freeman et al. 2001).
Disease causes significant losses of the marketable production; in mango can cause losses between 39 and 50 % in harvest and 52 % or more in postharvest (Páez Redondo, 2003; Kumari & Singh, 2017). In postharvest, the infective process is regulated by the length of the quiescent stage of the fungus during immature stages of fruits and it is generally activated when organs mature (Prusky et al. 2013; Ploetz, 2007).
The infectious process varies among species of Colletotrichum, and the occurrence of quiescence is one of those differential aspects. In thale cress (Arabidopsis thaliana), C. higginsianum cause leaf Anthracnose and once the appressorium is activated, the germinated appressorium forms a biotrophic primary hyphae and the fungus invades the epidermal cells without causing any damage and confined to the first invaded host cell; subsequently, a necrotrophic secondary hypha is formed and hemibiotrophic infective process takes place, invading new cells and intercellular spaces (O’Connell et al. 2012). In tomato - C. gloeosporioides interaction, melanized appressorium are formed from conidia germinated, and maintain quiescent during some time; after, they develop a penetration peg and differentiate to a dendritic like structure inside the fruit cuticle, still without penetration of the epidermal cells (Alkan et al. 2015); swollen hyphae within the epidermal cell were formed, these structures are also described in leaf infections by Colletotrichum species (O’Connell et al. 2012; Latunde-Dada, 2001). Usually, as the fruit ripen quiescence ends and infective hyphae extending from the swollen hyphae were observed; at this stage, the fungus shift to a necrotrophic phase (Alkan et al. 2015; O’Connell et al. 2012; Latunde-Dada, 2001).
Quiescence is part of the colonization process of several Colletotrichum species and occurs once the biotrophic phase begins in immature organs or during transition from the biotrophic to the necrotrophic stage (Prusky et al. 2013), in different hosts such as mango (Páez Redondo, 2003), tomato (Alkan et al. 2015), papaya (Amaral et al. 2017), avocado (Fischer et al. 2017), guava (Guédez & Rodríguez, 2021) and bananas (De Lapeyre de Bellaire et al. 2000), among others. Initial advances in transcriptomic studies have revealed the role of genes that are activated during the interaction between the fungus and some of its hosts, including quiescence. The amount and type of quiescence-related genes vary with the fungus species and the host involved, although some effectors and transcription factors are common (Fu et al. 2022; Korn et al. 2015; Alkan et al. 2015; O’Connell et al. 2012). However, the understanding of Colletotrichum species quiescence phase requires further research for different hosts, especially under tropical conditions. Therefore, this review aims to update the knowledge about quiescence in some Colletotrichum species in tropical fruit trees.
MATERIALS AND METHODS
The present paper consists of a literature review and provides information about the quiescence of Colletotrichum spp. in fruit species, and factors associated with its occurrence and breaking are explicitly highlighted, mainly under subtropical conditions. Additionally, concepts that could be the basis of questions or hypotheses for future research are raised, especially for tropics where advances on the subject are still limited.
The information consulted was taken from different scientific articles, review articles and chapter of books available in different biliographic databases such as Science Direct, Elsevier, Scopus and Google Scholar, emphasizing the aspects related to the occurrence of quiescence in the Colletotrichum-host interactions. Some keywords used were quiescence, Colletotrichum, quiescent infections, tropical fruit trees, Anthracnose, plant diseases, plant pathogens, management and endophytes, in different combinations of searches. No specific time period was defined for the search for information, to identify advances in knowledge on the topic; however, papers from the last 35 years were consulted, in English and Spanish.
RESULTS AND DISCUSSION
General aspects of quiescence. Quiescence is considered part of pre-colonizing process in some species of plant pathogens and is usually considered a resting stage of the microbe following the onset of infection, but before the necrotrophic colonization is initiated (Prusky et al. 2013). Quiescence is strategic for some phytopathogenic fungi, and occurs among biotrophic and necrotrophic stages of some hemibiotrophic pathogens (Figure 1); for some phytopathogenic fungi, it is not easy to distinguish whether if their lifestyle is hemibiotrophic or totally necrotrophic (Rajarammohan, 2021) and in this situation, quiescence can be the difference between both lifestyles. Quiescence is reported for other pathogens fungi-host interactions, such us grapevine (Vitis vinifera) - Neofusicoccum parvum (Czemmel et al. 2015), apple (Malus domestica) - Phlyctaena vagabunda (Lattanzio et al. 2001), pistachio (Pistacia vera) - Botryosphaeria dothidea (Ahimera et al. 2003), V. vinifera - B. cinerea (Haile et al. 2017), papaya (Carica papaya) - Lasiodiplodia theobromae (Amaral et al. 2017), mango (Mangifera indica) - Lasiodiplodia thebromae and Alternaria alternata (Diskin et al. 2017), mango - Botryosphaeria, Phomopsis and Pestalotiopsis (Johnson et al. 1992). This article will only focus on Colletotrichum-host interactions.
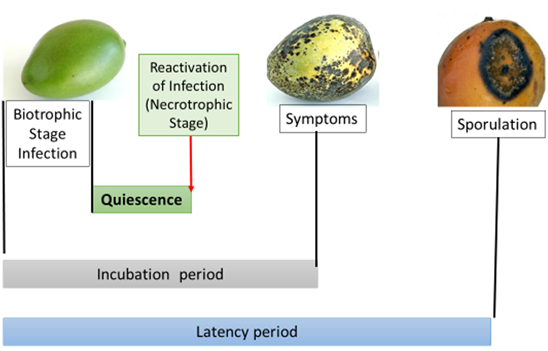
Figure 1 Scheme of an infective process by Colletotrichum sp. Quiescence after the biotrophic infective process has started; infection is reactivated and the fungus in its necrotrophic stage causes symptoms (incubation period) and subsequently produces a new inoculum (latency period).
Quiescent stages of Colletotrichum are reported in various fruit trees (Sharma & Kulshrestha, 2015; Prusky et al. 2013), such as banana and others Musaceae (Photita et al. 2005), guava (Psidium guajava) (Fischer et al. 2019), avocado (Persea americana) (Binyamini & Schiffmann-Nadel, 1972), mango (Mangifera indica) (Paramasivan et al. 2009), papaya (Carica papaya) (Siddiqui & Ali, 2014), passion fruit (Passiflora edulis f. flavicarpa and P .edulis f. edulis) (Joy & Sherin, 2016) and citrus trees (Citrus spp.) (Peres et al. 2005). Quiescence in the genus Colletotrichum is reported for some species, mainly in C. gloeosporioides specie (Prusky et al. 2013).
Quiescence is modulated by the availability of nutrients, metabolic changes, and presence of antifungal compounds in the host (Prusky et al. 2013), as well as to the insufficient enzyme potential of the fungus to invade unripe fruit (Adikaram et al. 2015). Furthermore, quiescence can be a strategy of Colletotrichum to avoid toxic levels of antifungal compounds present in unripe fruits (Adikaram et al. 2015). In this way, the fungus escapes the action of management strategies, and remains as an inoculum to infect at any time during harvest and postharvest.
The significant changes in metabolic synthesis and biochemical responses of the host fruit are regulated by differential genes expression that has been reported during the quiescent stage (Korn et al. 2015; Alkan et al. 2015; O’Connell et al. 2012). Genes that regulate infectious process of Colletotrichum are expressed differentially at each stage (Alkan et al. 2015; O’Connell et al. 2012; Latunde-Dada, 2001). The genes related to quiescence also differ according to the fungus species and infected host (Liu et al. 2022; Li et al. 2017;Korn et al. 2015; Alkan et al. 2015; Gan et al. 2013; O’Connell et al. 2012). During appressoria development, a significant up-regulation of factors modulating the melanization of fungal cell wall is expressed and is concomitant with the accumulation of glycerol, factors that contribute to appressorial strength and the process of highly localized turgor pressure (Alkan et al. 2015). Also, cyclic adenine monophosphate (AMP) and the mitogenic activation protein kinase (MAPK) signaling and other pathways, known to play a role in inducing appressoria formation and melanin biosynthesis in Colletotrichum, are up-regulated (Shnaiderman et al. 2013). During quiescence of C. gloeosporioides, 178 genes could be defined as quiescent-specific, and are related with cell cycle components that regulated the DNA synthesis, genes encoding for a G1/S-specific cyclin, histone modifiers, and ATP-dependent chromatin remodeling complexes regulating DNA accessibility needed for gene activation/repression (Alkan et al. 2015). The production of ammonia during Colletotrichum colonization and alkalization of the environment has been previously reported as factors that break quiescence (Shnaiderman et al. 2013; Alkan et al. 2013a; Miyara et al. 2010).
In simultaneous analysis of tomato - C. gloeosporioides interaction, fruit transcripts were highly up-regulated during the quiescent stage. Defense pathways were further up regulated, including the phenylpropanoid pathway for phytoalexin and lignin. Pathways for the synthesis of phenylpropanoid derivatives as potential substrates for peroxidase activity, was also increased in the expression. These results have shown that phytoalexin biosynthesis and lignification comprise a major ongoing defense pathway used by the fruit in response to the quiescent fungi (Alkan et al. 2015). Transcript expression changes from rishitin to the α‐tomatine biosynthetic pathway, suggesting an effective response to C. gloeosporioides, creating a hostile environment that would signal the fungus to enter an extended quiescent stage (Alkan et al. 2015).
In C. fructicola - pear leaves interaction, the key regulators of plant Salicylic Acid-mediated defense response, were mainly upregulated at QS (Fu et al. 2022).
These findings indicate that quiescence is not a simple resting stage of the pathogen, also host gene response may contribute to activate some pathways before the necrotrophic colonization is initiated.
Effect of host physiological changes on Colletotrichum quiescence. Hormone ethylene is a factor that modulates appressoria formation in the host; during quiescence pathogen stimulate fruit ethylene production and defensive responses, so will also enhance the ripening process and accelerate the release from quiescence (Prusky & Sionov, 2021). Exposure of the spores of C. gloeosporioides and C. musae to ethylene, at </=1 microl/liter, caused germination, branching of the germ tube, and formation of multiple appressoria from a single spore; propylene can induce spore germination and the formation of multiple appressoria, as well; ethylene induced germination and appressorium formation in the Colletotrichum sp. climacteric fruit but not in non-climacteric ones as citrus (Flaishman & Kolattukudy, 1994). While these results strongly suggest that this fruit fungal interaction have coevolved to develop a mechanism to use the host's ripening hormone as a signal to differentiate structure of infection; the fact is that processes do not occur concurrently and all appressoria formation for quiescence occurs in the orchard, away from the climacteric process where major ethylene quantities are produced (Prusky et al. 1996).
The breaking of quiescent stages includes a series of reactions that synchronize a decrease in the host’s defense with the acceleration of the infective process by the fungus. This includes cuticle degradation, cell wall degradation, fruit softening, increase in total soluble solids, changes in pH, decrease in preformed antifungal and inducible compounds, increase in reactive oxygen species (ROS) and hormones changes (Prusky & Sionov, 2021; Prusky et al. 2013). All these physiological changes associated with the fruit ripening create specific signals of fungal recognition for the initiation of the necrotrophic stage. Postharvest pathogens produce and secretes proteins and enzymes, which modify host cell walls, to overcome the physical constraints imposed by the wall and to release sugars to sustain its own energy needs. These processes might facilitate the intercellular expansion of hyphae and, thus, enhance the pathogen’s ability to access cellular resources. On the other hand, use of the wall's sugar constituents for energy might require the complete breakdown of a variety of polysaccharides into monosaccharides (Prusky et al. 2016b). The degradation of the plant cell wall matrix by pathogens may affect the proteins embedded in the cell wall and are likely to activate PAMP-triggered immunity (Mengiste, 2012), which often leads to callose deposition at sites of penetration, accumulate phenolic compounds and various toxins in the cell wall and synthesize lignin-like polymers to reinforce the wall (Hückelhoven, 2007). Because fruit cell wall degrading enzymes (CWDEs) are normally activated during ripening, it was commonly assumed that fruit softening contributes to the transition to susceptibility to pathogens (Paniagua et al. 2014; Blanco-Ulate et al. 2013). However, in some cases fungal colonization occurs also in unripe fruits (Wang et al. 2007), suggesting the complexity of the changes modulating quiescence. Also, plants PG inhibiting proteins (PGIPs) may reduce the pathogen pectin degradation (De Lorenzo et al. 2001). For this reasons inhibition of cell wall softening usually delay fruit susceptibility.
The alkalization of the environment tissue round the quiescent infection by ammonia may contribute to breaking quiescence by activation of fungal transcription factors that modulate the synthesis of pectolytic enzymes. These pH changes are modulated by nutritional availability in unripe or ripening fruits (Prusky & Ziv, 2019; Ment et al. 2015; Prusky et al. 2016a).
Chemical Compounds regulates quiescence in Colletotrichum-host interaction. Quiescence in Colletotrichum could be regulated by the production of antimicrobial compounds, production of phytoalexins, new enzymes and even the formation of physical resistance structures (Sanzani et al. 2012). Chemical compounds from immature organs of bananas (De Lapeyre de Bellaire et al. 2000), avocados (Ahimera et al. 2003) and mangoes (Kienzle et al. 2014) determine the maintenance of quiescence of some Colletotrichum species. Relationship between quiescence and high levels of antifungal compounds in the peel of immature fruits of some fruit trees such as mango and avocado has been described (Beno-Moualem & Prusky, 2000; Droby et al. 1986; Cojocaru et al. 1986).
The accumulation and reduction of antifungal compounds, mainly derived from natural lipids such as 1-acetoxy-dihydroxy-n-heptadecane-16-ene and others with single or triple double bonds, are responsible for the occurrence of quiescence or breakdown of it (Prusky & Ziv, 2019; Prusky et al. 2013); Recently, it has been shown that in the skin of Hass avocado fruits during ripening and storage, the persin and epicatechin contents decrease significantly (Bowen et al. 2018), which is related to the loss of quiescence of pathogens at maturity. These compounds have been specifically identified in immature avocado fruits, and inhibited germ tube elongation of Colletotrichum sp. conidia (Guetsky et al. 2005). The synthesis of resorcinols, gallotannins and chitinases in immature fruits is related to the quiescence of the pathogen; mango unripen fruits have shown a low susceptibility to Colletotrichum (Parthasarathy et al. 2015; Karunanayake et al. 2011; Paramasivan et al. 2009). In addition, the presence of potential sources of secondary metabolites such as n-hexane in the peel of immature fruits and latex of mango helps maintain the fungal quiescence (Sinniah et al. 2012).
Reactive oxygen species (ROS) synthesized by the host play a fundamental role in limiting the development of pathogens and maintaining their quiescence, because they modulate host’s resistance; generally, their increment is associated with hydrogen peroxide increase or addition in the epidermis of immature fruits, where the levels of phenylalanine ammonia lyase (PAL) and epicatechin are also higher (Prusky et al. 2013). Accumulation of ROS are the result of the balance between ROS production and antioxidant activity. In grape and tomato, ROS, lipid peroxidation, and protein oxidation were increased at breaker stage conditions where the fungus was still in a quiescent stage; in many fruits, storage is associated with an increase in ROS, which results either from increased ROS production or from a decrease in antioxidative activity (Hodges, 2003). Antioxidants may inhibit fruit ripening and senescence (Lester, 2003), while high O2 or application of H2O2 leads to increase ROS and senescence (Tian et al. 2013). However post-harvest pathogens may modulate host ROS production by the secretion of elicitors, toxins, and antioxidants to modify the plant ROS production (Aver’Yanov et al. 2012; Alkan et al. 2013a; Alkan et al. 2013b).
Pathogen development and break of quiescence. The breaking of the quiescence is synchronically regulated by fungal effector proteins that manipulate their hosts for effective colonization. The pathogen must maintain host viability during initial biotrophic and quiescent interaction, but after needs to elicit host death for subsequent necrotrophic development. This elicitation process occurs in ripening fruits concurrently or as result of host ripening. Most fungal effector genes are host-induced and expressed in consecutive waves associated with pathogenic transitions, indicating distinct effector suites are deployed at each stage. Kleemann et al. (2012) demonstrated that antagonistic effectors either induce or suppress plant cell death. Based on these results obtained in an interaction with a non-host plant such as Arabidopsis thaliana, it can be concluded that the quiescence of a hemibiotrophic species of Colletotrichum is guided through a coordinated expression of antagonistic effectors that support cell viability or death that is probably modulated by metabolic changes in the host.
Internal metabolic factors of the pathogen that modulate appressorial formation and colonization, also induce breakdown of quiescence. Ammonium secreted and or its accumulation by C. gloeosporioides during spore germination and host colonization contribute to pathogenicity (Prusky & Sionov, 2021; Prusky & Ziv, 2019; Ment et al. 2015; Prusky et al. 2016a; Prusky et al. 2016b; Miyara et al. 2010). AMET and MEPB genes modulate the secretion and accumulation of ammonia by the pathogen; in an investigation, the disruption of these genes resulted in 45 % less colonization of C. gloeosporioides in avocado fruits (Shnaiderman et al. 2013). The factors that active this nitrogen metabolism are still unknown, but it has been reported that nitrogen-metabolism genes GDH2, GS1, GLT, and MEP are differentially expressed during colonization by C. gloeosporioides in avocado (Miyara et al. 2010).
Another factor reported as an inducer of appressoria formation was the surface wax of the host; avocado (Persea americana) fruit wax induced germination and appressorium formation from the spores of C. gloeosporioides (Flaishman et al. 1995; Podila et al. 1993). On the other hand, the infective process of Colletotrichum species includes the degradation of the cuticle and cuticular waxes through cutinases, favoring the establishment of the pathogen and its penetration into the host's tissues (Villafana & Rampersad, 2020; Alkan et al. 2015).
Quiescence and endophytic behavior of Colletotrichum species. Endophytic stage refers to a behavior or habitat type of a microbe, which does not generate apparent damage or symptom expression within its plant host despite being active in the tissues during part of its life cycle (Hyde & Soytong, 2008) and they are generally located in the intercellular spaces behaving as endosymbionts (Rodriguez et al. 2009). On the other hand, quiescence corresponds to a biotrophic stage of halted infection in which induction of symptoms does not occur, waiting for the right time of host response (Prusky et al. 2013). Colletotrichum as endophytic or quiescent pathogen is related to asymptomatic tissues, but subsequently may change to necrotrophic pathogen due to signals in the host, with the consequent expression of symptoms in them (Ranathunge & Sandani, 2016; Delaye et al. 2013; Kogel et al. 2006). It has been suggested that quiescence may be a form of endophytic behavior or survival strategy on non-host species.
Pathogenicity of the species C. asianum, C. cliviae, C. dianesei (synonym C. melanocaulon), C. fructicola, C. karstii, C. tropicale and C. endomangiferae corresponding to endophytic isolates from asymptomatic mango tissues, was confirmed on leaves and fruits of this same plant species (Vieira et al. 2014), indicating that endophytic stage may be a quiescence-like strategy. In others research, it has been shown that several strains of Colletotrichum spp. obtained as endophytes in several plant species have resulted pathogenic by artificial inoculations, such as Phaseolus vulgaris (Gonzaga et al. 2015) and tropical grasses (Manamgoda et al. 2013).
These reports raise the discussion if the quiescent stage could be part of endophytic style of some Colletotrichum species, since they do not generate visible infections during phenological stages where the tissues are immature. Recent studies indicate that C. tropicale isolated from symptomless leaves and stems of mango cultivar Azúcar was pathogenic on ripen fruits (Quintero-Mercado et al. 2019). The study of endophytic species and the relationship of quiescence with this lifestyle of pathogenic species of Colletotrichum are part of the new focus of research, which will improve efficiency in the management of “Anthracnose”.
Management of Colletotrichum spp. as quiescent fungi. Quiescent structures constitute a hidden source of inoculum that remains invisible mainly in flowers and fruits (Ploetz, 2007). In recent research, it was found that quiescent structures of Colletotrichum sp., were present in asymptomatic tissues of leaves and stems of mango cv. Azúcar (Quintero-Mercado et al. 2019). This way, the fungus can escape the action of chemical molecules and even the effect of cultural practices. When conditions are favorable for infection, the fungus leaves the quiescent stage and start necrotrophic phase in plant tissues; thus, that any management measure is unsuccessful (Páez Redondo, 2003).
Traditionally, the technological options implemented for the management of fruit Anthracnose consist in reducing the active inoculum in the field, avoiding conditions for re-infections through sanitation, pruning, the use of drainages in the soil, and application of fungicides (Páez Redondo, 2003). Conventional postharvest management based on hot water, fungicides, and lately chitosan, affect the inoculum from the field located on the surface of the fruits (Kumari & Singh, 2017; Chowdappa et al. 2014; Prusky et al. 2013); however, the quiescent or endophyte inoculum is not significantly affected. Therefore, this article highlights strategies focused on affecting quiescent structures of the fungus, and that can be applied in field and postharvest.
The use of a wrapper impregnated with a mixture of chitosan-vanillin and zeolite is proposed as packaging for fruits, which allows the excess of ethylene to be absorbed. Although the results in mango Nam Dok Mai were positive, its use must be validated commercially (Jaimun & Sangsuwang, 2019).
The combination of chitin, microbial suspensions and thermal treatments could reduce the expression of Anthracnose in postharvest; in mango cv. Azúcar, heat treatment, microbial activity and storage at 13 °C of the fruits, allowed a reduction of quiescent infections between 83 and 89 % (Zapata-Narváez et al. 2021).
The use of preformed antifungal compounds is an option that should be considered for postharvest management of Anthracnose; these compounds are naturally synthesized in early phenological stages of the fruit (Adikaram et al. 2017). In mango, higher levels of gallotannins, resorcinols and chitinases have been found in resistant genotypes; gallotannins activity decreased with fruit ripening but this decreased is 10 % less in resistant cultivars (Paramasivan et al. 2009). Recent research focuses on evaluating the effect of volatile compounds produced by microorganisms and plants (Cheng et al. 2022; Huang et al. 2021; Shi et al. 2021), which need to be evaluated under field and postharvest conditions.
Treatments of mango fruits with microorganisms is an option that is being evaluated in postharvest, specially bacteria that could prevent the activation of quiescent stage. Pseudomonas fluorescens, Bacillus subtilis, Saccharomyces cerevisiae, Brevundimonas diminuta, Stenotrophomonas maltophilia, Candida membranifaciens and yeasts induce the synthesis of lytic enzymes, chitinase and glucanase, which has resulted in a longer quiescence period and reduction of Anthracnose severity during storage (Vivekananthan et al. 2004). However, the use of biological control is still uncertain. On the other hand, the search for metabolites that affect the synthesis of compounds by the pathogen or that degrade cellular components of the fungus must be deepened.
The induction of resistance can be considered in the control of infections from quiescent stages. Banana fruits harvested from plants treated with salicylic acid (SA) and acibenzolar-S-methyl, showed reduced Anthracnose severity and delayed onset of disease symptom expression (Wanigasekara et al. 2014). Another compound that has been used in post-harvest treatments is Butyl Hydroxyanisole (BHA), which was evaluated in mango fruits through vacuum infiltration 72 hours prior to C. gloeosporioides inoculation; a significant reduction in the incidence and size of the lesion was observed, which was correlated with an improvement in the contents of peroxidases, polyphenolxidases, chitinases, phenyl-alamin-liases, glucanases, hydrogen peroxide and phenolic compounds in fruits (Zhu et al. 2008).
Colletotrichum as well as Alternaria alternata, secrete ammonium and alkalinize the medium for the infective process. The immersion of mango fruits in hydrochloric acid (50 mM) alone delays the activation of quiescent Alternaria infections (Prusky et al. 2006). Acidification of the cell matrix is an option to consider in the short term for the treatment of infections from the quiescence of the fungus, however the use of fungicides in acidic solutions requires further investigation.
In conclusion, publications to date are clear and deep on infectious processes focused on the necrotrophic phase of the fungus, with good support from genomic studies on the interaction with the host. But, many aspects of the biology of quiescence and its expression in different Colletotrichum species, especially in mangoes and other tropical fruit crops, are still unknown. It is necessary to deepen the study of the relationship between endophytism and quiescence. In pathogenic endophytic species, such as C. gloeosporioides, the occurrence of quiescence can be considered a form of transition from endophytism to the necrotrophic phase, or could quiescence be considered a form of endophytism?
Holistic management of Anthracnose requires that we focus on how to manage the pathogenesis of an endophytic and quiescent fungus.















