Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
TecnoLógicas
Print version ISSN 0123-7799On-line version ISSN 2256-5337
TecnoL. no.31 Medellín July/Dec. 2013
Artículo de Revisión/Review Article
Anaerobic Digestion Modeling: from One to Several Bacterial Populations
Modelización de la Digestión Anaerobia: de una a Varias Poblaciones Bacterianas
Iván D. Ramírez-Rivas1
1PhD. Bioprocesses Engineering, Facultad de Ingeniería, Universidad del Quindío, Armenia-Colombia, idramirez@uniquindio.edu.co
Fecha de recepción: 08 de julio de 2013 / Fecha de aceptación: 17 de septiembre de 2013
Abstract
Anaerobic digestion systems are complex processes that unfortunately often suffer from instability causing digester failure. In order to be able to design, optimizing and operate efficiently anaerobic digestion systems, appropriate control strategies need to be designed. Such strategies require, in general, the development of mathematical models. The anaerobic digestion process comprises a complex network of sequential and parallel reactions of biochemical and physicochemical nature. Usually, such reactions contain a particular step, the so called rate-limiting step which, being the slowest, limits the reaction rate of the overall process. The first attempts for modeling anaerobic digestion led to models describing only the limiting step. However, over a wide range of operating conditions, the limiting step is not always the same. It may depend on wastewater characteristics, hydraulic loading, temperature, etc. It is apparent that the "limiting step hypothesis" leads to simple and readily usable models. Such models, however, do not describe very well the digester behavior, especially under transient operating conditions. This work reviews the current state-of-the-art in anaerobic digestion modeling. We give a brief description of the key anaerobic digestion models that have been developed so far for describing biomass growth systems, including the International Water Association’s Anaerobic Digestion Model 1 (ADM1) and we identify the areas that require further research endeavors.
Keywords: Anaerobic digestion, modeling, ADM1, inhibition, rate-limiting step, Waste Activate Sludge (WAS).
Resumen
Los sistemas de digestión anaeróbica son procesos complejos que desafortunadamente a menudo sufren de inestabilidad. A fin de diseñar, optimizar y operar eficientemente estos sistemas es necesario desarrollar estrategias apropiadas de control. Estas estrategias requieren, en general del desarrollo de modelos matemáticos. El proceso de digestión anaerobia comprende una red compleja de reacciones secuenciales y paralelas de naturaleza bioquímica y fisicoquímica. Generalmente, estas reacciones contienen un paso en particular, denominado reacción-limitante el cual siendo el más lento, limita la tasa de reacción del proceso global. Los primeros intentos del modelado de la digestión anaerobia condujeron a modelos que describen este paso limitante. Sin embargo, en una amplia gama de condiciones de funcionamiento, el paso limitante no siempre es el mismo. Este puede depender de las características de las aguas residuales, de la carga hidráulica, de la temperatura, etc. Es evidente que la "hipótesis del paso limitante" conduce a modelos simples y fácilmente utilizables. Sin embargo, estos modelos no describen muy bien el comportamiento del digestor, especialmente en condiciones transitorias de funcionamiento. Este trabajo revisa el estado del arte en modelización de la digestión anaerobia. Damos una breve descripción de los modelos clave de digestión anaerobia que se han desarrollado hasta el momento para describir sistemas con crecimiento de biomasa, incluyendo el modelo de digestión anaerobia No 1 (ADM1) de la Asociación Internacional del Agua (IWA) e identificamos las áreas que requieren esfuerzos futuros de investigación.
Palabras clave: Digestión anaerobia, modelización, ADM1, inhibición, etapa de reacción limitante, lodos activados residuales.
1. Biochemical process
Anaerobic digestion is a collection of processes by which microorganisms break down biodegradable material in the absence of oxygen. Anaerobic digestion is widely used as a source of renewable energy. The process produces a biogas, consisting of methane (CH4) and carbon dioxide (CO2), as well as trace gases like hydrogen sulfide (H2S) and hydrogen (H2) (Boe, 2006). This biogas can be used directly as fuel, in combined heat and power gas engines or upgraded to natural gas-quality bio-methane.
Extracellular solubilization steps are divided into disintegration and hydrolysis of which the first is a largely non-biological step and converts composite particulate substrate to particulate carbohydrates, protein, and lipids. The second is enzymatic hydrolysis and it is in three parallel processes to convert particulate carbohydrates, proteins and lipids into monosaccharides (MS), amino acids (AA), and long-chain fatty acids (LCFA), respectively, by using enzymes secreted by the micro-organisms to permit its transport through the cellular membrane. Once in the cell, these simple molecules can be used as energy source for the metabolism. In a next stage, monomers resulting from hydrolysis, as well as dissolved compounds are used as substrates by fermentative microorganisms, which mainly transform them into low molecular weight acids like Volatile Fatty Acids (VFAs) such as acetate, propionate, butyrate, and valerate, alcohols such as methanol and ethanol; and gases like CO2 and H2.
Degradation of higher organic acids to acetate is an oxidation step, with no internal electron acceptor. Therefore, the organisms oxidizing the organic acid (normally bacteria) are required to utilize an additional electron acceptor like hydrogen ions or carbon dioxide to produce hydrogen gas or formate, respectively. These electron carriers must be maintained at a low concentration for the oxidation reaction to be thermodynamically possible and hydrogen and formate are consumed by hydrogenotrophic methanogenic organisms. The thermodynamics of syntrophic acetogenesis and hydrogen utilizing methanogenesis reactions are only possible in a narrow range of hydrogen or formate concentrations (and also influenced to a lesser degree by other products and substrate concentrations).
Acetogenic bacteria produce acetate and hydrogen from acids that contain three or more carbon atoms in their structure. Acetogenesis from propionate, butyrate, and ethanol are thermodynamically unfavorable under standard conditions (ΔGo > 0) and they become possible only at very low partial H2 pressures (lower than 10-4 ppm) (Fukuzaki et al., 1990; Lee & Zinder, 1988). This requires the bacteria oxidizing the acids to work in syntrophy with hydrogenotrophic species, like methanogens, which by consuming hydrogen, maintain low partial pressure and enable these reactions to occur. The non-syntrophic acetogens mainly produce acetate and can also use CO2 as final electron acceptor. These bacteria are strictly anaerobic and are divided into two groups: fermentative acetogens (Pseudomonas, Clostridium, Ruminococcus) and hydrogenotrophic acetogens or homoacetogens (Acetogenium, Acetobacterium, Clostridium), which consume CO2 and H2. Finally, the acetic acid and the gas couple CO2/H2 are converted into CH4 by archaea called aceticlastic methanogens and hydrogenotrophic methanogens respectively (Ahring & Westermann, 1987).
The anaerobic wastewater treatment process presents very interesting advantages compared to the classical aerobic treatment (Mata-Alvarez & Llabres, 2000): It has a high capacity to degrade concentrated and difficult substrates (plant residues, animal wastes, food industry wastewater, and so forth), produce low amounts of sludge, requires little energy and in some cases, can even recover energy by using methane combustion. But in spite of these advantages, the anaerobic treatment plants often suffer from instability. Such instability is usually witnessed as a drop in the methane production rate, a drop in the pH, a rise in the volatile fatty acid (VFA) concentration, causing digester failure. It is caused by (a) feed overload, (b) feed under load, (c) entry of an inhibitor, or (d) inadequate temperature control. The usual remedy is a rapid increase in the hydraulic retention time (HRT) and when this fails, the digester has to be primed with sludge from a "healthy" digester. This, however, may be quite costly in view of the fact that anaerobic digestion is a very slow process due to the low growth rate of anaerobes microorganisms.
The most common reactor type used for anaerobic digestion of wastewaters is the Continuously Stirred Tank Reactor (CSTR). The main problem of this reactor type, i.e., the fact that the active biomass is continuously removed from the system leading to long retention times, has been overcome in a number of systems based on immobilization of the active biomass, henceforth referred to as high-rate systems. High-rate anaerobic reactors are becoming increasingly popular for the treatment of various types of wastewater because of their low initial and operational costs, smaller space requirements, high organic removal efficiency and low sludge production, combined with a net energy benefit through the production of biogas. The Up-flow Anaerobic Sludge Blanket reactor (UASB) and Anaerobic Filters (AF) are the most frequently used high-rate anaerobic reactors (Rajinikanth et al., 2008).
2. Anaerobic digestion models
Anaerobic digesters often exhibit significant stability problems that may be avoided only through appropriate control strategies. Such strategies generally require the development of appropriate mathematical models, which adequately represent the key processes that take place. This work reviews the current state-of-the-art in anaerobic digestion modeling, including the International Water Association’s Anaerobic Digestion Model 1 (ADM1) and to identify areas that require further research endeavors.
Dynamic modeling of anaerobic digestion has been an active research area during the last four decades. Andrews (1968) introduced the Haldane model to characterize biomass growth with substrate inhibition that can emphasize the process instability: A model with a single bacterial population (aceticlastic methanogens) was then proposed (Graef & Andrews, 1974). Usually, a process like anaerobic digestion contains one particular step, the so-called rate-limiting or rate-determining step, which, being the slowest, limits the rate of the overall process (Hill & Barth, 1977). In the Graf and Andrews model the conversion of fatty acids into biogas is considered limiting; according to this model, a digester is expected to fail whenever, for some reason, the fatty acid concentration is increased. This causes a drop in pH and a rise in un-dissociated acetic acid concentration. This, in turn, causes a drop in the growth rate of the methanogenic population, until they are washed out, if the situation is prolonged. This model can also predict the digester response to the entry of an external inhibitor.
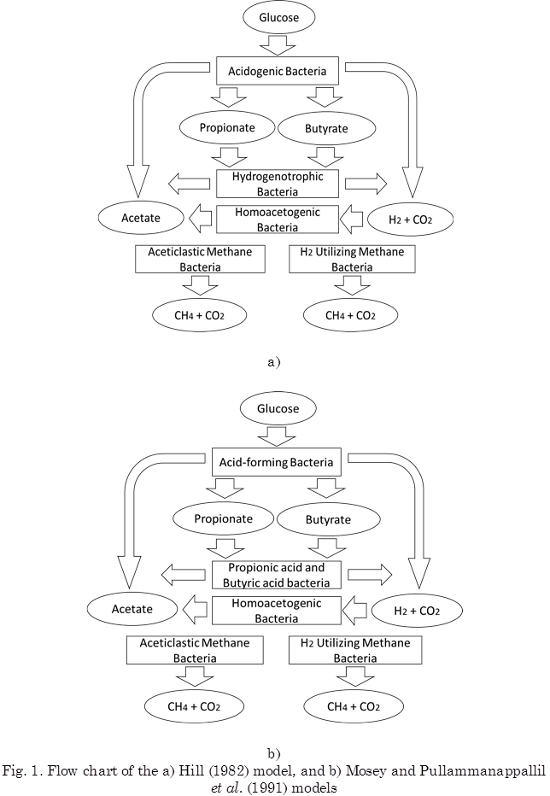
Hill (1982) introduced a model that was specially developed to describe digestion of manure and animal wastes. The model assumes that methanogenesis depends on the total fatty acids and inhibition by the total fatty acid concentration. The five bacterial groups assumed to participate in the overall digestion process are depicted in Fig. 1a). All five steps are assumed to be inhibited by high fatty acid concentrations. According to this model, anaerobic digestion is stalled, whenever an accumulation of VFAs is brought about. In particular, inhibition causes a decrease in the rate of VFA consumption, leading to acid accumulation. Above a certain critical VFA concentration, the digester fails regardless of the pH value.
Mosey (1983) introduced a four-population model with one acidogenic reaction, one acetogenic reaction, and two methanation reactions, which also empathizes the role of hydrogen, as shown in Fig. 1b. The fatty acid relative production is assumed to depend on the redox potential or equivalently on the (NADH) / (NAD+) ratio (Nicotinamide Adenine Dinucleotide). This ratio is made a function of the hydrogen partial pressure in the gas phase. According to the Mosey model, a sudden increase in the organic loading rate is expected to cause an accumulation of VFAs, given that acetogens grow at a slower rate than the acidogens. The subsequent drop in pH inhibits the hydrogen-utilizing methanogenic bacteria, causing a rise in the hydrogen partial pressure, which causes further accumulation of propionic and butyric acids. Methane generation is stalled when pH drops to particularly low levels (< 5.5).
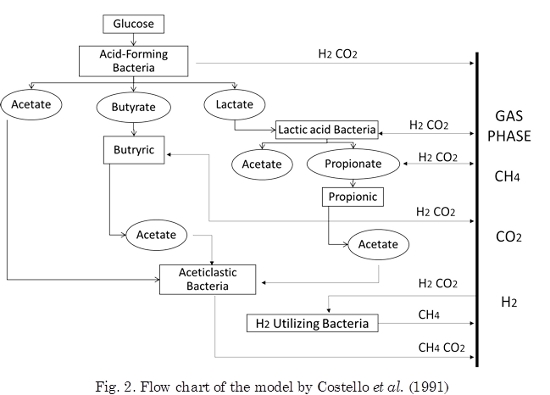
Based on the work of Mosey, two models were developed (Pullammanappallil et al., 1991; Costello et al., 1991a, 1991b). The Pullammanappallil’s model allowed describing the gas phase and acetoclastic inhibition by undissociated fatty acids. Costello assumed that glucose is first converted into acetic, butyric, and lactic acids, followed by conversion of lactate into propionate and acetate by another bacterial group (Fig. 2).
All the models described thus far, although capable of predicting digester failure caused by a specific disturbance, either through a drop in pH and/or through accumulation of volatile fatty acids (a commonly observed behavior in digesters treating municipal sludge and/or high organic content industrial wastewaters), none could adequately describe anaerobic digestion of manure (Angelidaki, 1992), given that digesters fed with manure exhibit self-regulation of the pH, attributed to the ammonia generated.
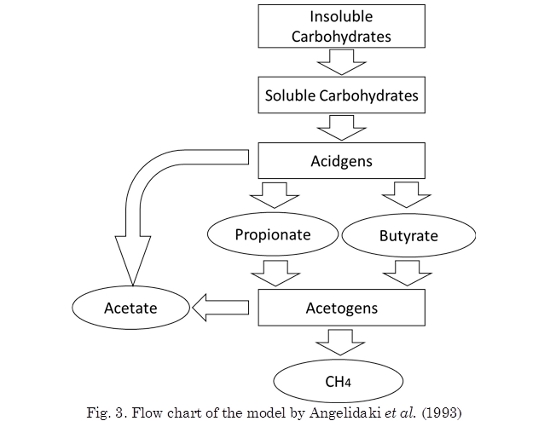
The model by Angelidaki et al., (1993), considers hydrolysis, acidogenesis, acetogenesis, and methanogenesis (Fig. 3) and it is very good for describing the behavior of manure-fed digesters. In this model, free ammonia is assumed to inhibit methanogenesis; acetic acid is assumed to inhibit acetogenesis; and total VFA is assumed to inhibit acidogenesis. The maximum specific growth rate of the bacteria and the degree of ionization of ammonia are assumed to depend on temperature and pH.
The pH self-regulation mechanism is as follows. Whenever free ammonia (high for high pH) inhibits methanogenesis, acetic acid is accumulated. This causes an inhibition for acetogenesis, and a consequent accumulation of propionic and butyric acids, leading to inhibition of acidification. VFA accumulation reduces the pH, causing a decrease in the free ammonia concentration and the inhibition of methanogenesis. The process is, thus, self-regulatory, unless the magnitude of the disturbance is larger than the system can withstand. When this occurs, the pH drops significantly and causes digester failure.
Nevertheless, all models described so far consider organic matter as a whole and do not account for the nature of the organic macromolecules in the feed composition. A modeling approach that takes the complex feed composition (breakdown in carbohydrate, protein, VFAs, and other organics) into account was proposed by Gavala et al., (1996). This model was capable of adequately predicting the Chemical Oxygen Demand (COD) and fatty acid dependence on the operating conditions, and should be useful for designing co-digestion processes of agricultural industrial wastewater, but the model does not take into account the particular nature of the developed granular sludge in high-rate systems (Hulshoff, 1989), like biofilm reactors: Up-flow Anaerobic Sludge Bed Reactor (UASBR), Expanded Granular Sludge Bed (EGSB) reactor, Anaerobic biofilter, Anaerobic Fluidized Bed Reactor (AFBR), or an Anaerobic Baffled Reactor (ABR). In developing kinetic models for both UASBRs and AFBRs, granule structure plays an important role. Studies show that the structure of the granules and bacterial composition depends on the type of effluent being treated (Cooper & Sutton, 1983). Various theories are provided to support the layered and un-layered structures of the granules. Incorporation of this variation in the models poses a challenge for the modelers (Heijnen, et al., 1989; Saravanan & Sreekrishnan, 2006; Lopez et al., 2013).
3. The IWA anaerobic digestion model: ADM1
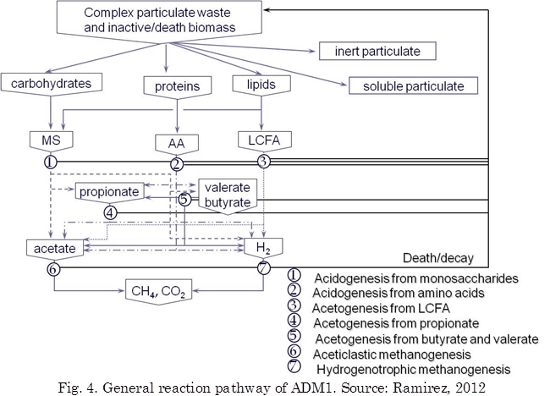
The International Water Association (IWA) task group for mathematical modeling of anaerobic digestion process developed a common model: ADM1 that can be used by researches and practitioners (Batstone et al., 2002; Ozkan-Yucel & Gokcay, 2010; García-Gen et al., 2013). ADM1 is an excellent simulation platform given its adequate structure that is able to handle many different situations encountered experimentally. The ADM1 model is a structured model that reflects the major processes involved in the conversion of complex organic substrates into CH4 and CO2 and inert byproducts. Fig. 4 presents an overview of the substrates and conversion processes addressed by the model. It is not the aim of this work to recite the many interesting reactions and phenomena occurring in the Anaerobic Digestion (AD) process. However, for clarity, in the following an overview of the AD process, as seen in many recent textbooks, is given.
In ADM1, both biochemical as well as physicochemical processes is included. All components except inorganics are expressed in terms of their COD. Nitrogenous species and inorganic carbon species are described in terms of their molar concentrations. The biochemical reaction pathway includes: (i) an extracellular disintegration step converting composite particulate matter into carbohydrates, lipids and proteins, (ii) an extracellular enzymatic hydrolysis step converting the degradation products into their chemical building blocks, i.e. monosaccharides (MS), long chain fatty acids (LCFA) and amino acids (AA), (iii) acidogenesis or fermentation of the building blocks into hydrogen, acetate and volatile fatty acids (VFA), i.e. propionate, butyrate and valerate, (iv) acetogenesis of VFA to acetate, (v) acetoclastic and hydrogenotrophic methanogenesis. Additionally, the death of biomass is taken into account.
To address these mechanisms, the model employs 26 state variables to describe the behavior of soluble (represented with a capital ‘‘S’’) and particulate components (represented with a capital ‘‘X’’). In addition the model addresses inorganic carbon (carbon dioxide and bicarbonate) and nitrogenous species (ammonia and ammonium). All of the species that dissociate as a function of pH (VFAs and ammonia) have variables defined for both the protonated and non-protonated species (Parker, 2005). The model maintains a charge balance among ionic species and, hence, there are variables for inorganic anions and cations including the hydrogen ion. The model solves for the hydrogen ion concentration and, thereby, the pH by ensuring chemical neutrality in the solution. Particulate species consist of either active biomass species or particulate substances incapable of directly passing through bacterial cell walls. In Fig. 4 particulate species are those with a capital ‘‘X’’. The microbial species considered in the model include sugar fermenters (Xsu), amino acid fermenters (Xaa), LCFA oxidizers (Xfa), butyrate and valerate oxidizers (Xc4), propionate oxidizers (Xpro), aceticlastic methanogens (Xac), and hydrogenotrophic methanogens (Xh2). Non-microbial particulate species include complex organics that either enters the process in the influent or that result from the death and decay of microbial species and the products of disintegration of the complex organics. This latter group consists of carbohydrates, proteins, and LCFAs.
The disintegration of Xc and hydrolysis of Xch, Xpr, and Xli are described by first-order rate expressions. Substrate conversion processes are described by Substrate-based uptake Monod-type that is used as the basis for all intracellular biochemical reactions. Death of biomass is represented by first-order kinetics and it is maintained in the system as composite particulate material. In the model, inhibition functions include pH (all groups), hydrogen (acetogenic groups), and free ammonia (aceticlastic methanogens). Liquid–gas mass transfer of gaseous components (CH4, CO2, and H2) is described by mass transfer relationships.
The ADM1 is a tool that allows predictions of sufficient accuracy to be useful. Because of the varying demands in process development, operation, and optimization, a different degree of model calibration and validation will be required in each case (Batstone et al., 1997, Ramirez, 2012).
ADM1 clarified the diverse previous approaches of anaerobic modelers (which at heart were very similar) into a model with common units, structure, and a base parameter set. It is a mechanistic model and has justifiably been criticized as being overly complicated, with difficulty in characterizing inputs and parameters, but it achieved its goals of a coordinated model, and to diversify the user base of anaerobic modeling. In fact, many of the weaknesses of anaerobic digestion modeling (i.e., poor inputs, and over-parameterization) were exposed due to a whole new group of anaerobic digestion modelers entering the field, largely from the activated sludge modeling (and whole-plant) field. Entry of these experts into the field also accelerated entry of new researchers, given that a number of cross-verified implementations were published and made freely available. The most popular of these is probably the Matlab implementation (Rosen & Jeppsson, 2006), which has moved through several iterations, and has been used worldwide in multiple published studies. The main other models in use apart from the ADM1 (or derivatives) are simplified models developed for specific applications.
In order to include spatial considerations within the ADM1 model, so that it allows us to have gradients of diversity in reactors with different configurations, several somewhat simplified distributed parameter models of the anaerobic digestion process have already been proposed. In the studies by Kalyuzhnyi et al., (2006) and Schoefs et al., (2004), relatively simple reaction kinetics was used. Batstone et al., (2005) developed a distributed parameter model by combining the ADM1 kinetics with the Takacs clarifier model (Takacs et al., 1991), which approximates a UASB reactor by using several layers, i.e., reactor hydrodynamics was simplified. In contrast, Mu et al., (2008) presented a comprehensive distributed parameter model, which combines the biotransformation kinetics of ADM1 with the axial dispersion transport model. They used a hyperbolic tangent function to describe biomass distribution within a one-compartment model. They showed that similar simulation results are obtained when this approach was compared with a two-compartment model, which consisted of a sludge bed and a liquid above the bed compartments, (the one-compartment model had less equations). It should be noted that the hyperbolic tangent model of the sludge bed does not take into account physical processes of granule settling and washout, but provides a nonlinear regression model of the experimentally measured sludge distribution. This regression model should be substituted by more-complex models (e.g., Kalyuzhnyi et al., 2006) if some insight on granular sludge dynamics is desired.
3.1 ADM1 limitations
Anaerobic digestion modeling is a rapidly developing area, with a tremendous scope in terms of quality, topic, and applicability. It is becoming more mainstream and as more expert modelers apply and develop anaerobic digestion, principles of good modeling, calibration, and evaluation practice from the aerobic-activated sludge field are equally applicable to anaerobic digestion. In the next section, we outline some of the key developments in the last years, as well as required areas of research.
Initial work with ADM1 (to 2005) was reviewed in a workshop in Copenhagen (Batstone et al., 2006), and a number of specific limitations were identified, including: glucose fermentation models, physicochemical system modeling, input characterization, parameter variation and validation in a broader context. Many of the 30 papers presented at this workshop addressed some of these limitations, and subsequent work has significantly advanced in at least the second two areas. Additional areas, including external electron acceptors (nitrate and sulfate), electron transfer, and inhibitor and toxicant behavior have active research communities, and continue to be developed.
In the two last World Congresses in Anaerobic Digestion (2007-2010), completely novel areas, including increases in complexity to represent model diversity were developed (Ramirez & Steyer, 2008; Ramirez et al., 2009), as well as application of ADM fundamentals to microbial fuel cell modeling (Picioreanu et al., 2010; Rodríguez et al., 2006), however, four key limitations still remain:
Glucose fermentation modeling received a partial boost with the publication of a new theoretical model by Rodríguez et al.,(2007). This has been partially validated and further developed by the same group, but it is evident that there is still no clear picture of how to represent glucose fermentation in a generalized way. From the hydrogen production perspective, fermentation modeling has decreased in importance, due to the possibility of thermal and electrochemically assisted hydrogen production, direct from glucose and acetate (Liu et al., 2005).
The physicochemical system used in anaerobic digestion modeling is fairly sophisticated, but it has proven to be inadequate for complex and non-dilute systems. In particular, key limitations mean that divalent ions are particularly poorly represented, which causes problems for modeling of key states, including phosphate (González-Cabaleiro et al., 2013). This has really not been addressed well and it is becoming a key issue, especially because physicochemical system modeling is being increasingly applied in activated sludge modeling, sensors, alternative systems (e.g., anaerobic ammonium removal, microbial fuel cells), and pure physicochemical systems (e.g., anion removal by precipitation).
Inputs and interfacing are a recognized issue in anaerobic digestion. In the last ten years, a number of approaches have been proposed, with most based on maintaining continuity of the major elemental and charge compounds. Generalized continuity based interface models (CBIM) have been proposed and widely applied by the Ghent team (Vanrolleghem et al., 2005; Volcke et al., 2006; Zaher et al., 2007). These models emphasize continuity of elements Carbone, Hydrogen, Nitrogen, Oxygen and Phosphate (CHNOP) and charge. The key issue is that the user must eliminate degrees of freedom when the destination side has more input states than the source side. This is very much the case for almost any model for the ADM1. CBIM principles can also be applied to input models and this has been done for general wastewaters (Kleerebezem & Van Loosdrecht, 2006), primary sludge (Huete et al., 2006), and solid waste (Zaher & Chen 2006; Nopens et al., 2007). Iterative or stepwise CBIM model is a type of tailored CBIM model that removes the problem of excessive degrees of freedom on the destination side by using knowledge of the specific system (e.g., primary sludge, or ASM1 states to ADM1). Much of this work has been done by the Benchmarking Task group to interface ASM1 and ADM1 states. Copp et al., (2003) proposed the first type of this model, while Nopens et al., (2007) proposed an updated version, which has also been used as an input model (Batstone et al., 2006). These interface models have evolved significantly in terms of applicability and accuracy (Ikumi et al., 2013). The key issue is now probably expanding the user interface by publishing the code, and increasing robustness.
Initial parameter validation post publication was mainly on primary sludge. This has now moved onto diverse systems, and further validation of parameters under special conditions (e.g., sulfate reduction). The applicability of ADM1 parameters on primary and activated sludge has become more widely accepted, such that Modeling has become benchmark of reactor performance (i.e., model parameters represent the majority of well-functioning systems), particularly for activated primary sludge (Sotemann et al., 2006). Model outputs are currently more limited by input characterization than kinetic parameters (i.e., stoichiometrically controlled for well-functioning systems).
Recent work with ADM1 (to 2013) was reviewed in a 13th world congress on AD in Santiago de Compostela (Spain). The last achievements are in the topics of control and monitoring of AD process. However, a survey of the recent trends in Monitoring and Control of Anaerobic digestion process is subject to another research work.
4. Conclusions
Anaerobic digestion is a very complex process involving various bacterial populations and substrates. With the progresses in instrumentation and in computer science, the development of mathematical models, predicting the dynamic process behavior has attracted considerable attention in the last two decades. ADM1 is undoubtedly one of the milestones of this research era. However, modeling is always a goal-driven exercise, and many alternative models have been proposed in the literature, depending on the aim, e.g., process understanding, dynamic simulation, optimization, or control.
Models contain unknown parameters, e.g., initial conditions, stoichiometry, and kinetic parameters which have to be estimated from experimental data. Parameter identification is a delicate task due the potentially large number of parameters and the scarcity of informative experimental data. This review attempts to summarize the efforts that have been accomplished in anaerobic digestion modeling, including the International Water Association’s Anaerobic Digestion Model 1 (ADM1) and to identify areas that require further research endeavors and also highlights one particular step, in each model, the so-called rate-limiting or rate-determining step, which, being the slowest, limits the rate of the overall process.
5. References
Ahring, B.K. and Westermann P. (1987). Kinetics of butyrate, acetate, and hydrogen metabolism in a thermophilic, anaerobic butyrate-degrading triculture. Appl. Environ. Microb., 53, 434-439. [ Links ]
Andrews J. (1968). A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol. Bioeng. 10, 707-723. [ Links ]
Angelidaki, I. (1992). Anaerobic thermophilic biogass process: the effect of lipids and ammonia, Ph.D. Thesis, Technical University of Denmark, Copenhagen. [ Links ]
Angelidaki, I., Ellegaard, L., and Ahring, B.K. (1993). A mathematical model for dynamic simulation of anaerobic digestion of complex substrates: focusing on ammonia inhibition, Biotechnology and Bioengineering, 42, 159-166. [ Links ]
Batstone D.J.; Picioreanu C., and van Loosdrecht M.C.M. (2006). Multidimensional modelling to investigate interspecies hydrogen transfer in anaerobic biofilms, Water research, 40(16), 3099-108. [ Links ]
Batstone D., Keler J., Newell B., and Newland M. (1997). Model development and full scale validation for anaerobic treatment of protein and fat waste water. Water Sci and Technol., 36, 423-43. [ Links ]
Batstone D.J., Keller J., Angelidaki I., Kalyuzhnyi S.V., Pavlostathis S.G., Rozzi A., Sanders W.T.M., Siegrist H., and Vavilin V.A. (2002). Anaerobic Digestion Model No 1. Scientific and technical report 13, International Water Association (IWA), London. [ Links ]
Batstone, D.J., Gernaey, K.V., Steyer, J.P., and Schmidt, J.E. (2005). A particle model for UASB reactors using the Takacs Clarifier Model. In: Proceedings of the First International Workshop on the IWA Anaerobic Digestion Model No. 1, Copenhagen, Denmark, 129-136. [ Links ]
Batstone, D.J., Keller, J., and Steyer, J.P. (2006). A Review of ADM1 Extensions, Applications, and Analysis 2002-2005, Water science and technology, 54(4), 1-10. [ Links ]
Boe, K. (2006). On-line monitoring and control of the biogas process, PhD. thesis, Technical University of Denmark, Lyngby, Denmark. [ Links ]
Cooper, P.F., and Sutton, P.M. (1983). Treatment of wastewaters using biological fluidized beds, Chemical Engineering, vol. 393, 392-405. [ Links ]
Copp, J., Jeppsson, U., and Rosen, C. (2003). Towards an ASM1-ADM1 state variable interface for plant-wide wastewater treatment modeling". In: Proceedings 76th Annual WEF Conference and Exposition, Los Angeles, USA. [ Links ]
Costello, D.J., Greenfield, P.F., and Lee, P.L. (1991). Dynamic modeling of a single-stage high-rate anaerobic reactor- I. Model derivation, Wat. Res., 25, 847-858. [ Links ]
Costello, D.J., Greenfield, P.F., and Lee, P.L. (1991). Dynamic modeling of a single-stage high-rate anaerobic reactor- II. Model verification, Wat. Res., 25, 859-871. [ Links ]
Fukuzaki S., Nishio N., Shobayashi M., and Nagai S. (1990). Inhibition of the fermentation of propionate to methane by hydrogen, acetate and propionate. Appl. Environ. Microb., 56, pp.719-723. [ Links ]
García-Gen, S., Lema, J.M. and Rodríguez, J. (2013). ADM1 application to anaerobic co-digestion: generalised implementation of fermentable soluble substrates. Proceeding of the 13th world congress on Anaerobic Digestion. Santiago de Compostela, Spain, June 25-28, 2013. [ Links ]
Gavala, H.N., Skiadas, I.V., Bozinis, N.A., and Lyberatos, G. (1996). Anaerobic codigestion of agricultural industries wastewaters, Wat. Sci. Tech., 34, 67-75. [ Links ]
González-Cabaleiro, R., Lema, J. M., Rodríguez, J. and Kleerebezem, R. (2013). Linking thermodynamics and kinetics to assess pathway reversibility in anaerobic fermentations. Proceeding of the 13th world congress on Anaerobic Digestion. Santiago de Compostela, Spain, June 25-28, 2013. [ Links ]
Graef, S.P. and Andrews, J.F. (1974). Stability and control of anaerobic digestion. Journal WPCF, 46, 667-682. [ Links ]
Heijnen, J.J., Mulder, A., Enger, W., and Hoeks, F. (1989). Review on the application of anaerobic fluidized bed reactors in wastewater treatment, Chemical Engineering Journal, 4, B37-B50. [ Links ]
Hill, D.T. and Barth, C.L. (1977). A dynamic model for simulation of animal waste digestion. Journal of Water Pollution Control Federation, 49(10), 2129-2143. [ Links ]
Hill, D.T. (1982). A comprehensive dynamic model for animal waste methanogenesis, Transaction of the ASAF, 25, 2129-2143. [ Links ]
Huete, E., De Gracia, M., Ayesa, E., and Garcia-Heras, J.L. (2006). ADM1-based methodology for the characterization of the influent sludge in anaerobic reactors, Water Science and Technology, 54(4), 157-166. [ Links ]
Hulshoff Pol, L.W. (1989). The phenomenon of granulation of anaerobic sludge. Ph.D. Thesis, Agricultural University Wageningen, the Netherlands. [ Links ]
Ikumi, D.S., Harding T.H. and Ekama, G.A. (2013). Plant wide wastewater treatment modelling - biodegradability of organics. Proceeding of the 13th world congress on Anaerobic Digestion. Santiago de Compostela, Spain, June 25-28, 2013. [ Links ]
Kalyuzhnyi, S.V., Fedorovich, V.V., and Lens, P. (2006). Dispersed plug flow model for upflow anaerobic sludge bed reactors with focus on granular sludge dynamics, J. Ind. Microbiol. Biotechnol., 22, 221-237. [ Links ]
Kleerebezem, R. and Van Loosdrecht, M.C.M. (2006). Waste characterization for implementation in ADM1, Wat. Sci. Technol., 54(4), 167-174. [ Links ]
Lee M.J. and Zinder S.H. (1988). Hydrogen partial pressure in a thermophilic acetate-oxydizing methanogenic coculture, Appl. Environ. Microb., 54, 1457-1461. [ Links ]
Liu, H., Grot, S., and Logan, B.E. (2005). Electrochemically assisted microbial production of hydrogen from acetate", Environ. Sci. Technol., 39, 4317-4320. [ Links ]
López, I., Odriozola, M. and Borzacconi, L. (2013). Modelling diffusional effects in anaerobic granules and its consequences on reactor design. Proceeding of the 13th world congress on Anaerobic Digestion. Santiago de Compostela, Spain, June 25-28, 2013. [ Links ]
Mata-Alvarez J. and Llabres P. (2000). Anaerobic digestión of organic solid wastes. An overview of research achievements and perspectives, Bioresour. Technol., 74, 3-16. [ Links ]
Mosey, F.E. (1983). Mathematical modeling of the anaerobic digestion process: regulatory mechanisms for the formation of short-chain volatile acids from glucose, Wat. Sci. Technol., 15, 209-232. [ Links ]
Mu, S.J., Zeng, Y., Wu, P., Lou, S.J., and Tartakovsky, B. (2008). Anaerobic digestion model no. 1-based distributed parameter model of an anaerobic reactor: I. Model development. Bioresource technology., 99, 3665-3675. [ Links ]
Nopens, I., Sin, G., Jiang, T., d'Antonio, L., Stama, S., Zhao, J., and Vanrolleghem, P.A. (2007). Model-based optimisation of the biological performance of a sidestream MBR", Water Sci Technol., 56(6), 135-43. [ Links ]
Ozkan-Yucel, U.G., Gokcay, C.F. (2010). Application of ADM1 model to a full-scale anaerobic digester under dynamic organic loading conditions. Environmental Technology. 31(6), 633-640. [ Links ]
Parker W. J. (2005). Application of the ADM1 model to advanced anaerobic digestion, Biores. Technol., 96, 1832-1842. [ Links ]
Picioreanu C, van Loosdrecht MC, Curtis TP., and Scott K. (2010). Model based evaluation of the effect of pH and electrode geometry on microbial fuel cell performance, Bioelectrochemistry, 78(1), 8-24. [ Links ]
Pullammanappallil, P., Owens, J.M., Svoronos, S.A., Lyberatos, G., and Chynoweth, D.P. (1991). Dynamic model for conventionally mixed anaerobic digestion reactors, AIChE Annual meeting, paper 277c, 43-53. [ Links ]
Rajinikanth, R., Ramírez I., Steyer, J.P., Mehrotra I, Kumar P., Escudie R., and Torrijos M. (2008). Experimental and modeling investigations of a hybrid upflow anaerobic sludge-filter bed (UASFB) reactor, Wat. Sci. Tech., 58(1), 109-116. [ Links ]
Ramírez, I. and Steyer, J.P. (2008). Modeling microbial diversity in anaerobic digestion. Wat. Sci. Tech., 57(2), 265-270. [ Links ]
Ramírez, I. (2012). ADM1 applications for a hybrid up-flow anaerobic sludge-filter bed reactor performance and for a batch thermophilic anaerobic digestion of thermally pretreated waste activated sludge, Revista Facultad de Ingeniería, U de A., 65, 167-179. [ Links ]
Ramírez, I., Volcke, E.I.P., Rajinikanth, R., and Steyer, J.P. (2009). Modelling microbial diversity in anaerobic digestion thorough an extended ADM1 model, Water research., 43(11), 2787-2800. [ Links ]
Rodríguez, J., Kleerebezem, R., Lema, J.M., and van Loosdrecht, M. (2006). Modeling product formation in anaerobic mixed culture fermentations, Biotechnol. Bioeng., 93(3), 592-606. [ Links ]
Rodríguez, J., Rabaey, K., Blackall, L., Keller, J., Batstone, D., Verstraete, W., and Nealson, W.K. (2007). Microbial ecology meets electrochemistry: Electricity driven and driving communities, The ISME Journal, 1, 9-18. [ Links ]
Rosen C.; Vrecko D.; Gernaey K. V.; Pons M. N.; Jeppsson U. (2006). Implementing ADM1 for plant-wide benchmark simulations in Matlab/Simulink. Water Science and Technology, 54(4), 11-19. [ Links ]
Saravanan V. and Sreekrishnan T.R. (2006). Modellimg anaerobic biofilm reactors-A review. Journal of Environmental Management, 8(6), 1-18. [ Links ]
Schoefs, O., Dochain, D., Fibrianto, H., and Steyer, J.P. (2004). Modeling and identification of a partial differential equation model for an anaerobic wastewater treatment process. In: Proceedings of the 10th World Congress on Anaerobic Digestion, Montreal, Canada, 1, 343-347. [ Links ]
Sotemann, S.W., Van Rensburg, P., Ristow, N.E., Wentzel, M.C., Loewenthal, R.E., and Ekama, G.A. (2006). Integrated chemical, physical and biological processes modelling of anaerobic digestion of sewage sludge. Water Science and Technology, 54(5), 109-117. [ Links ]
Takacs, I., Patry, G.G., and Nolasco, D. (1991). A dynamic model of the clarification-thickening process. Water Res., 25, 1263-1271. [ Links ]
Tomei, M.C., Braguglia, C.M., Cento, G., Mininni, G., (2009). Modeling of anaerobic digestion of sludge. Critical Reviews in Environmental Science and Technology 39(12), 1003-1051. [ Links ]
Vanrolleghem, P.A., Rosen, C., Zaher, U., Copp, J., Benedetti, L., Ayesa, E., and Jeppsson, U. (2005). Continuity-based interfacing of models for wastewater systems described by Petersen matrices, Wat. Sci. Technol., 52, 493-500. [ Links ]
Volcke, E.I.P., Van Loosdrecht, M.C.M., and Peter A Vanrolleghem, P.A. (2006). Continuity-based model interfacing for plant-wide simulation: A general approach, Water Res., 40(15), 2817-2828. [ Links ]
Zaher, U. and Chen, S. (2006). Interfacing the IWA Anaerobic Digestion Model No.1 (ADM1) with manure and solid waste characteristics. WEFTEC.06, Conference Proceedings, Annual Technical Exhibition & Conference, 79th, Dallas, TX, United States, 3162-3175. [ Links ]
Zaher, U., Grau, P., Benedetti, L., Ayesa, E., and Vanrolleghem, P.A. (2007). Transformers for interfacing anaerobic digestion models to pre- and post-treatment processes in a plant-wide modeling context, Environmental Modelling & Software, 22(1), 40-58. [ Links ]