Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
TecnoLógicas
Print version ISSN 0123-7799On-line version ISSN 2256-5337
TecnoL. vol.20 no.40 Medellín Dec. 2017
Artículo de investigación/Research article
CO, Pb ++ and SO2 effects on L-type calcium channel and action potential in human atrial myocytes. In silico study
Efectos del CO, Pb ++, y SO2 en el canal de calcio tipo L y potencial de acción en miocitos auriculares humanos. Estudio en silico
Diana C. Pachajoa1, Catalina Tobón2, Juan P. Ugarte3, Javier Saiz4
1 Ingeniera Biomédica, Facultad de Ciencias Exactas y Aplicadas, Instituto Tecnológico Metropolitano, Medellín-Colombia, dianacaterin@gmail.com
2 PhD en Ingeniería Electrónica, Grupo de investigación MATBIOM, Facultad de Ciencias Básicas, Universidad de Medellín, Medellín-Colombia, ctobon@udem.edu.co
3 MSc en Ingeniería, Facultad de Ingeniería, Universidad de San Buenaventura, Medellín-Colombia, rei_jpu@icloud.com
4 PhD en Ingeniería Industrial, Centro de Investigación e Innovación en Bioingeniería, Universidad Politécnica de Valencia, Valencia-España, jsaiz@ci2b.upv.es
Fecha de recepción:19 de junio de 2017/ Fecha de aceptación: 20 de agosto de 2017
Como citar / How to cite
D.C. Pachajoa, C. Tobón, J.P. Ugarte, y J. Saiz, CO, Pb++ and SO2 effects on L-type calcium channel and action potential in human atrial myocytes. In silico study. TecnoLógicas, Vol. 20, No. 40, pp. 113-123, 2017.
Abstract
Exposure to air pollutants like carbon monoxide (CO), lead (Pb++) and sulfur dioxide (SO2) promotes the occurrence of cardiovascular diseases. Experimental studies have shown that CO, Pb++ and SO2 block L-type calcium channels, reducing the calcium current (ICaL) and the action potential duration (APD), which favors the initiation of atrial arrhythmias. The goal is to study the effects of CO, Pb++ and SO2 at different concentrations on ICaL and action potential using computational simulation. For this purpose, models of the effects of the air pollutants on the atrial L-type calcium channel were developed and were incorporated into a mathematical model of a human atrial cell. The results suggest that CO, Pb++ and SO2 block the ICaL current in a fraction that increases along with the concentration, generating an APD shortening. These results are consistent with experimental studies. The combined effect of the three air pollutants produced an APD shortening, which is considered to be a pro-arrhythmic effect.
Keywords: Air pollution, atrial action potential, calcium channel, in silico models.
Resumen
La exposición a contaminantes atmosféricos, como el monóxido de carbono (CO), plomo (Pb++) y dióxido de azufre (SO2), promueve la aparición de enfermedades cardiovasculares. Estudios experimentales han demostrado que el CO, el Pb++ y el SO2 bloquean los canales de calcio tipo L, disminuyendo la corriente de calcio (ICaL) y la duración del potencial de acción (APD), favoreciendo el inicio de arritmias auriculares. El objetivo es estudiar los efectos del CO, Pb++ y SO2 a diferentes concentraciones, sobre ICaL y el potencial de acción auricular mediante simulación computacional. Para ello, se desarrollaron modelos matemáticos de los efectos de los contaminantes atmosféricos sobre el canal de calcio auricular tipo L y se incorporaron en un modelo matemático de células auriculares humanas. Los resultados sugieren que el CO, el Pb++ y el SO2, bloquean la corriente ICaL en una fracción que aumenta a medida que aumenta, la concentración, generando un acortamiento del APD. Estos resultados son consistentes con estudios experimentales. El efecto combinado de los tres contaminantes atmosféricos generó un acortamiento del APD, lo cual es considerado un efecto pro-arrítmico.
Palabras clave: Contaminantes atmosféricos, potencial de acción auricular, canal de calcio, modelos en silico.
1. Introduction
Air pollution is defined as the presence in the atmosphere of one or more substances in sufficient quantity to produce health alterations. Air pollution causes 4.3 million premature deaths annually [1]. In 2010, the economic cost of the impact of air pollution on health in developing countries was around USD 1.7 billion [1].
Carbon monoxide (CO) is a toxic gas that results from incomplete combustion. When people breath, the CO binds to hemoglobin and it is retained in the blood, significantly reducing the amount of oxygen that it can transport [2]. Lead (Pb++) is a toxic agent that can exert adverse effects on human health. According to the United States Environment Protection Agency (EPA) Pb++ is one of the most dangerous air pollutants, affecting multiple human body systems [3]. In general, more than 143.000 people die every year due to illnesses related to Pb++ [4]. Sulfur dioxide (SO2) is an invisible gas that has a nasty, sharp smell. The main source of SO2 in the air is industrial activity that involves processing materials that contain sulfur. It causes cardiovascular diseases and cardiovascular mortality [5].
Exposure to these air pollutants contributes to cardiovascular diseases [6]. Epidemiological studies have reported effects such as heart failure, generation of cardiac arrhythmias and decreased heart rate variability [3-13]. Experimental studies have shown that Pb++ blocks L-type calcium channels [14]. A decrease in L-type calcium current (ICaL) is an important mechanism that favors the generation of atrial arrhythmias [15]. Recently, it has been shown that chronic exposure to CO promotes a pathological phenotype of cardiomyocytes in which remodeling and intracellular Ca++ overload increase the risk of arrhythmias [10, 12] and ischemia [16]. On the other hand, experimental studies suggest that exposure to SO2 produces an effect on the ICaL current that can produce damage in cardiomyocytes, which in turn results in hypoxia and ischemia [17, 18]. The objective of this work is to study, through computer simulation, the electrophysiological effects of Pb++, CO and SO2 at different concentrations on ICaL and action potential handset under normal electrophysiological conditions.
2. Methodology
2.1 Human atrial cell model
The Courtemanche Ramirez Nattel Kneller [19, 20] membrane formalism was implemented to simulate the electrical activity of human atrial cell. The transmembrane voltage (Vm) is given by (1):
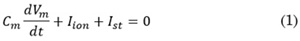
Where Cm is the membrane capacitance (100 pF), Iion is the total membrane current and Ist is the external stimulus current. The model is considered under normal electrophysiological conditions.
2.2 Model of CO effect on ICaL
Based on an experimental study [16], a relationship between CO concentration and APD decrease was established. This study attributes the APD decrease to a blockage of the ICaL current; therefore, a basic model of CO’s effect on the ICaL current was developed. The blocking factor (bco) of the ICaL current by CO depends on the concentration of CO (D) and has a lineal behavior. This equation was introduced in the ICaL equation of the cell model, and it was adjusted to obtain APD reductions approximately equal to those observed experimentally.
The equations to calculate bco and ICaL are given by Eq. (2) and (3):
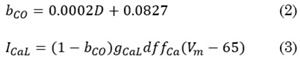
Where gICaL is the maximum conductance of ICaL, d is the activation gate, f is the voltage-dependent inactivation gate, fCa is the calcium-dependent inactivation gate and 65 is the value of equilibrium potential for ICaL.
2.3 Model of Pb++ effect on ICaL
To develop a basic model of the Pb++ effect on ICaL, the steady-state fraction of block (bPb) was used. Hill’s equation has been used to fit the concentration-response relationships for ligand block. It describes the fraction of the macromolecule saturated by ligand as a function of the ligand concentration; it is used for determining the degree of cooperativeness of the ligand binding to the receptor. In this model, the kinetics of the channel would be considered unchanged in the presence of the Pb++, Eq. (4).

Where IC50 is the half maximal inhibitory concentration for the current block by Pb++ and D is the Pb++ concentration. A Hill coefficient (h) of 1 indicates completely independent binding. A total of 152 nM was used for the IC50 to block ICaL; this value was found by [14] in ventricular myocytes. There are no reported values of IC50 for blocking channel by Pb++ on atrial myocytes.
The following is the modified equation to simulate the ICaL current
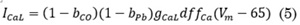
2.4 Model of SO2 effect on ICaL
Based on experimental studies [17] [18], a basic model of the SO2 effect on ICaL, current was developed. The decrease factor of ICaL, by SO2 (bSO2) depends on the SO2 concentration (D) [17], as follows, Eq. (6):

The modified equation to simulate the ICaL current is (7).
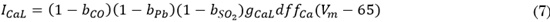
2.5 Simulation protocol and data analysis
Unicellular models to simulate the sinus rhythm under physiological conditions were implemented using the Cellular Open Resource public CellML OpenCOR® software. Forward Euler’s method, with a time step of 0.001 ms, was implemented to solve the equations. A train of 10 stimuli was applied. The basic cycle length was 1000 ms. Concentrations of CO from 0 to 1000 µM, of Pb++ from 0 to 300 nM and of SO2 from 0 to 200 µM were implemented.
APD at 90% of the repolarization (APD90) as well as ICaL and INA currents were measured. All measurements were made on the 10th beat using a program developed in MATLAB® software.
3. Results and discussion
3.1 CO effects on ICaL and action potential
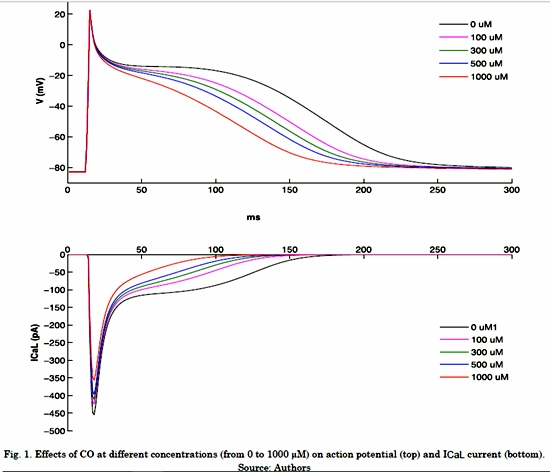
Fig. 1 shows the effects of different CO concentrations on the Fig. 1 shows the effects of different CO concentrations on the ICaL current and atrial action potential under normal electrophysiological conditions. It is possible to observe that ICaL without CO shows a peak of -449 pA and the current remains active during the plateau phase of action potential. As the CO concentration increases, ICaL downregulation is observed, which causes an APD shortening (Table 1) and loss of plateau phase. When the highest CO concentration was applied (1000 µM), the ICaL peak decreased 21% (-356 pA) and the APD90 reached a value of 151 ms, which indicates a decrease of 28%.
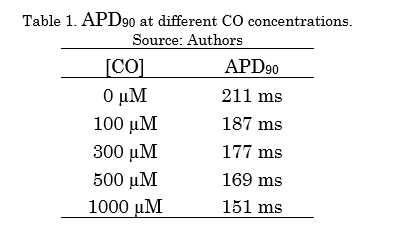
Table 1 shows the APD90 decreasing values as a function of the different CO concentrations. The increase in CO concentration generated an APD shortening.
3.2 Pb++ effects on ICaL and action potential
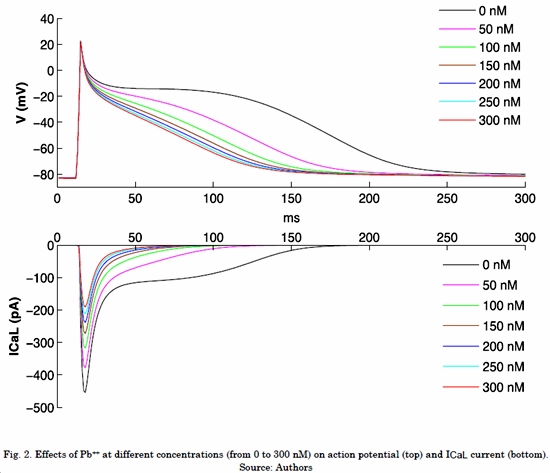
Fig. 2 shows the effects of different Pb++ concentrations on ICaL current and atrial action potential under normal electrophysiological conditions. It can be observed that ICaL without Pb++ shows a peak of -454 pA and the current remains active during the plateau phase of action potential. As with CO, while Pb++ concentration increases, ICaL downregulation is observed, which causes an APD shortening (Table 2) and loss of plateau phase. When the highest Pb++ concentration was applied (300 nM), the ICaL peak decreased 58% (-191 pA) and the APD90 reached a value of 115 ms, which indicates a decrease of 45%.
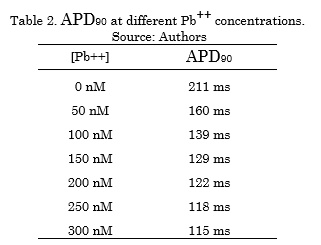
Table 2 shows the APD90 decreasing values as a function of the different Pb++ concentrations. The increase in Pb++ concentration generated an APD shortening.
3.3 SO2 effects on ICaL and action potential
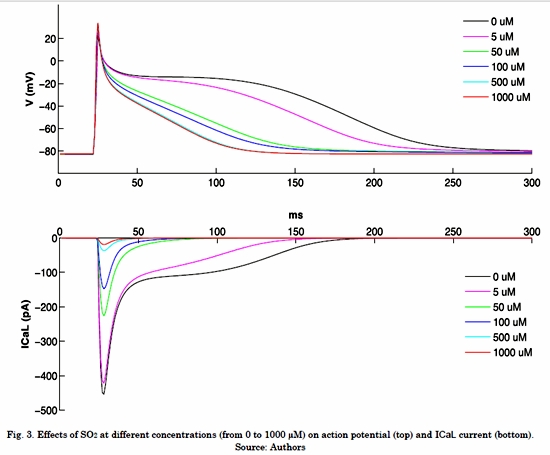
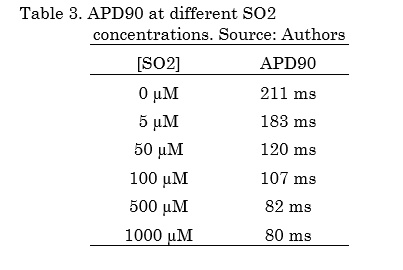
Fig. 3 shows the effects of different SO2 concentrations on ICaL, INa and atrial action potential under normal electrophysiological conditions. It is possible to observe that ICaL without SO2 shows a peak of -454 pA and this current remains active during the plateau phase of action potential. When the highest concentration of SO2 was applied (1000 µM), the ICaL peak creased 96% (-19.2 pA) and the APD90 reached a value of 80 ms, which indicates a decrease of 62% (Table 3).
3.4 SO2 effects on ICaL and action potential
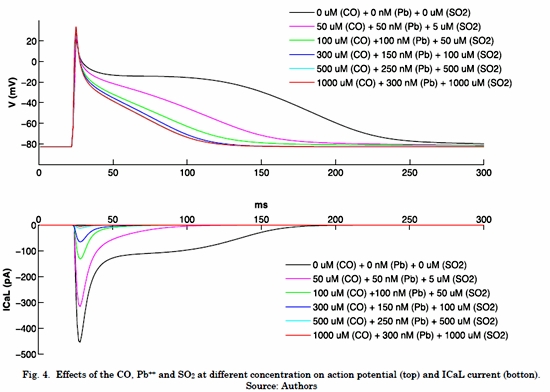
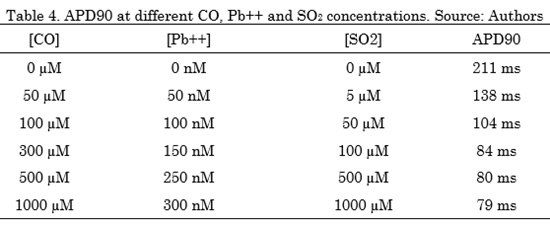
The results of this section show the combined effects of the three air pollutants CO, Pb++ and SO2, at different concentrations on the ICaL and action potential, under normal electrophysiological conditions. It can be observed in Fig. 4, that ICaL without pollutants shows a peak of -454 pA, this current remains active during the plateau phase of action potential. When the highest concentrations of CO (1000) Fig. 3. Effects of SO2 at different concentrations (from 0 to 1000 µM) on action potential (top) and ICaL current (bottom). Source: AuthorsµM), Pb++ (300 nM) and SO2 (1000 µM) where applied, the ICaL peak decreased 99% (-4.9 pA) and the APD90 reached a value of 79 ms, which indicates a decrease of 63% (Tabla 4). The effects of the three air pollutants in combination caused an important APD decrease.
Table 4 shows the APD90 decreasing values in function of the different concentration of the three air pollutants. CO, Pb++ and SO2 concentrations increases generate an APD shortening.
The results showed that CO blocks the ICaL current in a greater fraction as CO concentration increases. These results are consistent with experimental studies. A study in rats [16] showed that CO leads to an important reduction of the APD in atrial myocardium, as well as a significant acceleration of the sinus rhythm and reduction in the force of contraction. Another study in rats showed that CO inhibits native rat cardiomyocyte L-type Ca2+ currents [21]. Additionally, it has been demonstrated that CO pollution promotes cardiac remodeling and ventricular arrhythmia in healthy rats [22]. A recent study using mathematical simulations showed that CO causes early post-depolarizations in the ventricular action potential [23] due to effects on the sodium and calcium currents, which demonstrates its pro-arrhythmic effect. Our results are consistent with these studies: as soon as the concentration of CO increases, the APD shortening is observed under normal physiological conditions. There are no in silico studies of the effects of CO on human atrial action potential.
The results also showed that Pb++ blocks the ICaL current in a greater fraction as the concentration increases. This extends its action in time, which results in an APD shortening, as experimentally demonstrated [14]. This analysis is consistent with an experimental study of ventricle myocytes of rats [14]. In it, Pb++blocked the L-type calcium channels; however, its mechanism is not well explained. A study of the ventricular myocardium of rats suggests that acute lead administration reduces the myocardial contractility by reducing sarcolemmal calcium influx and the myosin ATPase activity [24]. It has been observed that Pb++ may alter the bioelectrical properties of neurons by blocking high-voltage activated Ca2+ currents, particularly L-type [25], and differentially blocking the cloned T-type calcium channels [26]. There are no in silico studies of the effects of Pb++ on human atrial action potential.
Similarly, the results showed that SO2 also blocks the ICaL current in a greater fraction as the concentration increases, which results in ADP reductions. Experimental studies of ventricular myocytes of rats [17, 18] showed that exposure to SO2 causes a decrease of the ICaL current and an increase of the INa current, which can produce damage in cardiomyocytes and result in hypoxia and ischemia. Our results are consistent with these studies: when the concentration of SO2 increases, the APD decreases under normal physiological conditions. There are no in silico studies of the effects of SO2 on human atrial action potential.
When the combined effects of the three air pollutants, CO, Pb++ and SO2, at different concentrations on ICaL were simulated, the results showed a significant APD decrease in a greater fraction as the concentrations increase, which is a severe pro-arrhythmic effect. Clinical studies have shown that air pollution increases the risk of cardiovascular disease mortality by 76 % [27]; such deaths are mainly related to ischemia, arrhythmias and heart failure [23-25]. Sufficient evidence has been found to conclude that a brief exposure to high levels of pollutants increases the patients’ cardiac mortality. Likewise, it has been shown that prolonged exposures reduce people's life expectancy by several years and hospital admissions due to cardiovascular diseases increase with high concentrations of pollutants [31]. Recent studies have been able to demonstrate that cardiac arrhythmias present higher probability of occurrence after the exposure to air pollutants, thus concluding that air pollution is an acute "trigger" of these arrhythmias [32]. Despite the existence of studies of the effects of air pollutants on the cardiovascular system in the literature, the mechanisms underlying the effects of acute and chronic exposure to these agents on the heart have not been well established. In silico studies may contribute to a better understanding of the mechanisms by which air pollutants have unhealthy effects on cardiac tissue, promoting cardiac diseases such as arrhythmias.
4. Conclusion
Our results are in agreement with experimental studies that have demonstrated that CO, Pb++ and SO2 block the ICaL current in a greater fraction as the concentration increases, which results in an APD shortening. It was possible to simulate the combined effects of the three air pollutants with models. The results showed an important APD decrease that suggests a relevant pro-arrhythmic effect.
This in silico study contributes with research into the electrophysiological effects of CO, Pb++ and SO2 on the atrial action potential of healthy people. To our knowledge, this is the first work that has developed mathematical models that evaluate the effect of air pollutants on human atrial currents and action potential.
References
[1] OECD, The Cost of Air Pollution. OECD Publishing, 2014. [ Links ]
[2] C. R. Henry, “Myocardial Injury and Long-term Mortality Following Moderate to Severe Carbon Monoxide Poisoning,” Jama, vol. 295, no. 4, p. 398, Jan. 2006. [ Links ]
[3] U.S. EPA, “Integrated Science Assessment (ISA) for Lead (Final Report, Jul 2013),” Washington, 2013. [ Links ]
[4] W. H. Organization, “Lead poisoning and health,” 2016. [Online]. Available: http://www.who.int/mediacentre/factsheets/fs379/en/ [ Links ]
[5] United States Environmental Protection Agency, “Air Quality Planning and Standards.,” 2016. [Online]. Available: https://www3.epa.gov/airquality/. [ Links ]
[6] A. Bhatnagar, “Cardiovascular pathophysiology of environmental pollutants,” Am. J. Physiol. Heart Circ. Physiol., vol. 286, no. 2, pp. H479--H485, 2004. [ Links ]
[7] S. J. Kopp, J. C. Baker, L. S. DAgrosa, and P. L. Hawley, “Simultaneous recording of his bundle electrogram, electrocardiogram, and systolic tension from intact modified Langendorff rat heart preparations I: Effects of perfusion time, cadmium, and lead,” Toxicol. Appl. Pharmacol., vol. 46, no. 2, pp. 475-487, Nov. 1978. [ Links ]
[8] R. C. Prentice and S. J. Kopp, “Cardiotoxicity of lead at various perfusate calcium concentrations: Functional and metabolic responses of the perfused rat heart,” Toxicol. Appl. Pharmacol., vol. 81, pp. 491-501, 1985. [ Links ]
[9] M. A. Ansari, Z. H. Maayah, S. A. Bakheet, A. O. El-Kadi, and H. Korashy, “The role of aryl hydrocarbon receptor signaling pathway in cardiotoxicity of acute lead intoxication in vivo and in vitro rat model,” Toxicology, vol. 306, pp. 40-49, 2013. [ Links ]
[10] E. Samoli, G. Touloumi, J. Schwartz, H. R. Anderson, C. Schindler, B. Forsberg, M. A. Vigotti, J. Vonk, M. Kosnik, J. Skorkovsky, and K. Katsouyanni, “Short-Term Effects of Carbon Monoxide on Mortality: An Analysis within the APHEA Project,” Environ. Health Perspect., vol. 115, no. 11, pp. 1578-1583, Nov. 2007. [ Links ]
[11] R. Dales, “Ambient Carbon Monoxide May Influence Heart Rate Variability in Subjects With Coronary Artery Disease,” J. Occup. Environ. Med., vol. 46, no. 12, pp. 1217-1221, 2004. [ Links ]
[12] X. Qin, Z. Qian, M. G. Vaughn, E. Trevathan, B. Emo, G. Paul, W. Ren, Y. Hao, and G. Dong, “Gender-specific differences of interaction between obesity and air pollution on stroke and cardiovascular diseases in Chinese adults from a high pollution range area: A large population based cross sectional study,” Sci. Total Environ., vol. 529, pp. 243-248, Oct. 2015. [ Links ]
[13] B. Z. Simkhovich, M. T. Kleinman, and R. A. Kloner, “Air Pollution and Cardiovascular Injury. Epidemiology, Toxicology, and Mechanisms,” Journal of the American College of Cardiology, vol. 52, no. 9. pp. 719-726, 2008. [ Links ]
[14] J. Bernal, J. H. Lee, L. L. Cribbs, and E. Perez-Reyes, “Full reversal of Pb++ block of L-type Ca++ channels requires treatment with heavy metal antidotes.,” J. Pharmacol. Exp. Ther., vol. 282, no. 1, pp. 172-80, Jul. 1997. [ Links ]
[15] S. Dinanian, C. Boixel, C. Juin, J.-S. Hulot, A. Coulombe, C. Rücker-Martin, N. Bonnet, B. Le Grand, M. Slama, J.-J. Mercadier, and S. N. Hatem, “Downregulation of the calcium current in human right atrial myocytes from patients in sinus rhythm but with a high risk of atrial fibrillation,” Eur. Heart J., vol. 29, no. 9, pp. 1190-1197, 2008. [ Links ]
[16] D. V Abramochkin, N. N. Haertdinov, M. V Porokhnya, A. L. Zefirov, and G. F. Sitdikova, “Carbon monoxide affects electrical and contractile activity of rat myocardium,” J. Biomed. Sci., vol. 18, no. 1, p. 40, 2011. [ Links ]
[17] R.-Y. Zhang, J.-B. Du, Y. Sun, S. Chen, H.-J. Tsai, L. Yuan, L. Li, C.-S. Tang, and H.-F. Jin, “Sulfur dioxide derivatives depress L-type calcium channel in rat cardiomyocytes,” Clin. Exp. Pharmacol. & Physiol., vol. 38, no. 7, pp. 416-422, Jul. 2011. [ Links ]
[18] A. Nie and Z. Meng, “Study of the interaction of sulfur dioxide derivative with cardiac sodium channel,” Biochim. Biophys. Acta - Biomembr., vol. 1718, no. 1-2, pp. 67-73, Dec. 2005. [ Links ]
[19] M. Courtemanche, “Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model,” Cardiovasc. Res., vol. 42, no. 2, pp. 477-489, May 1999. [ Links ]
[20] J. Kneller, R. Zou, E. J. Vigmond, Z. Wang, L. J. Leon, and S. Nattel, “Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties.,” Circ. Res., vol. 90, no. 9, pp. E73--87, 2002. [ Links ]
[21] J. L. Scragg, M. L. Dallas, J. a Wilkinson, G. Varadi, and C. Peers, “Carbon monoxide inhibits L-type Ca2+ channels via redox modulation of key cysteine residues by mitochondrial reactive oxygen species.,” J. Biol. Chem., vol. 283, no. 36, pp. 24412-24419, 2008. [ Links ]
[22] L. Andre, J. Boissière, C. Reboul, R. Perrier, S. Zalvidea, G. Meyer, J. Thireau, S. Tanguy, P. Bideaux, M. Hayot, F. Boucher, P. Obert, O. Cazorla, and S. Richard, “Carbon monoxide pollution promotes cardiac remodeling and ventricular arrhythmia in healthy rats,” Am. J. Respir. Crit. Care Med., vol. 181, no. 6, pp. 587-595, Mar. 2010. [ Links ]
[23] B. Trenor, K. Cardona, J. Saiz, S. Rajamani, L. Belardinelli, and W. R. Giles, “Carbon monoxide effects on human ventricle action potential assessed by mathematical simulations,” Front. Physiol., vol. 4, no. 282, pp. 1-11, 2013. [ Links ]
[24] D. V Vassallo, E. C. Lebarch, C. M. Moreira, G. A. Wiggers, and I. Stefanon, “Lead reduces tension development and the myosin ATPase activity of the rat right ventricular myocardium,” Brazilian J. Med. Biol. Res., vol. 41, no. 9, pp. 789-795, 2008. [ Links ]
[25] Z. Parvin, J. Mahyar, F. Seyed Mohamamd, and M. Fereshteh, “The effect of lead (Pb2+) on electrophysiological properties of calcium currents in F77 neuron in Helix aspersa,” Physiol Pharmacol, vol. 4, no. 2, pp. 145-160, 2000. [ Links ]
[26] S. Jeong, B. Park, J.-W. Lee, and J.-H. Lee, “Divalent metals differentially block cloned T-type calcium channels,” Neuroreport, vol. 14, no. 11, pp. 1537-1540, 2003. [ Links ]
[27] K. A. Miller, D. S. Siscovick, L. Sheppard, K. Shepherd, J. H. Sullivan, G. L. Anderson, and J. D. Kaufman, “Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women,” N. Engl. J. Med., vol. 356, no. 5, pp. 447-458, 2007. [ Links ]
[28] R. D. Brook, B. Franklin, W. Cascio, Y. Hong, G. Howard, M. Lipsett, R. Luepker, M. Mittleman, J. Samet, S. C. Smith, and I. Tager, “Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association,” Circulation, vol. 109, no. 21. pp. 2655-2671, 2004. [ Links ]
[29] M. M. Finkelstein, “Pollution-Related Mortality and Educational Level,” Jama J. Am. Med. Assoc., vol. 288, no. 7, pp. 830-830, Aug. 2002. [ Links ]
[30] C. A. Pope, R. T. Burnett, M. J. Thun, E. E. Calle, D. Krewski, K. Ito, and G. D. Thurston, “Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution.,” Jama, vol. 287, no. 9, pp. 1132-1141, 2002. [ Links ]
[31] United Nations Environment Programme, Air Pollution: Worlds Worst Environmental Health Risk. 2014. [ Links ]
[32] M. S. Link, H. Luttmann-Gibson, J. Schwartz, M. A. Mittleman, B. Wessler, D. R. Gold, D. W. Dockery, and F. Laden, “Acute exposure to air pollution triggers atrial fibrillation,” J. Am. Coll. Cardiol., vol. 62, no. 9, pp. 816-825, 2013 [ Links ]