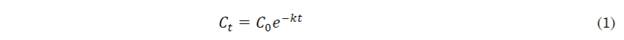1. INTRODUCTION
It is expected that the year 2025 will see sales of 246.20 billion dollars in the global market for bakery products, and the bread market will have revenue equaling 460.40 million dollars [1], [2]. The consumption of bread is high because it is used as a dietary source in various countries. In 2020, average per capita consumption was 24.50 kg, and the most consumed bread type is wheat because of its textural and sensory properties [2], [3]. In recent years, consumers became interested in bakery products with functional properties that contribute to their well-being and health, and this has resulted in a growth in the use of functional ingredients in bread production [3], [4].
Fruit by-products are a potential source for use in this industry because they are reservoirs of functional ingredients, such as fiber, minerals, and phytochemicals [5], [6]. Researchers have reported that phytochemicals are non-nutritive bioactive compounds found in plant matrices and are broadly classified as carotenoids, phenolics, alkaloids, nitrogen compounds, and organosulfur compounds [6], [7]. These researchers indicated that there is a negative correlation between the intake of phytochemicals and various diseases such as chronic inflammation, cardiovascular disease, cancer, and diabetes. Different studies have used fruit by-products as a source of carotenoid compounds in bakery products. [8] studied the mango epicarp in cookies, [9] evaluated the epicarp of tomato in bread, [10] investigated the epicarp of passion fruit in bread and cake, [11], and [12], studied mandarin epicarp as a color additive in bread. However, these studies did not evaluate the effect of temperature on carotenoids during the baking process.
In addition, [13]-[15] stated that the yield of baking processes is complex because the mechanisms of cooking processes, in particular chemical reactions, are not yet fully understood. They also indicated that studies on cooking are important to maintaining product quality during cooking. Kinetic and thermodynamic studies would be very helpful to predicting and modeling chemical reactions of bioactive compounds in bakery products during baking treatments, which has been studied in thermal treatments in other vegetable matrices [16]-[18]. Therefore, the objective of this research was to study the effect of temperature on the concentration of carotenoids and provitamin A when baking bread in a temperature range of 160-200 ºC.
2. MATERIALS AND METHODS
2.1 Obtaining the lipid extract from mandarin epicarp
Once mandarin epicarp flour was obtained according to [11], The samples were dehydrated with forced convection at a temperature of 40 ºC, for four hours, until obtaining a final humidity of 8 %, and the particle size of the dehydrated samples was reduced to 200 US mesh in an IKA Labortchnik grinding mill. The flour was subjected to extraction with ultrasound in sunflower oil, following the methodology described by [12]. The extraction was carried out with the following parameters: solid-liquid ratio of 0.0004 g/mL, one hour of extraction, temperature of 60 ºC, 240 W, and 42 kHz; the temperature was controlled with circulating water cooled at 2 ºC.
2.2 Bread with lipid extract from mandarin epicarp
The bread was obtained following the methodology of [12], with some modifications. The ingredients were: 300 g of wheat flour, 108 g of water, 74 g of margarine, 30 g of sugar, 30 g of eggs, 6 g of salt, 6 g of fresh yeast, and 20 g of lipid extract from mandarin epicarp, the amount of lipid extract is taken according to [12]. The process began by activating dry yeast in a mixture of water at 40 °C, sugar, and 10 g of flour for 10 min. Afterwards, the activated yeast was mixed with the rest of the ingredients in a Kitchenaid Artisan, USA blender, at level 6 for 15 min. The dough was left to rest for 15 min and divided into 60 g, making the dough into a circular shape. The dough was leavened at 30 °C for 40 min until it tripled in size. Once the proofing process concluded, the samples were baked in an Oster oven. The temperatures were 160, 180 and 200 ºC for 0, 10, 20, 30, 40, and 50 minutes. The temperature profiles in the oven, on the bread surface, and in the center of the bread were controlled with T-type thermocouples.
2.3 Determination of physicochemical parameters in bread
The moisture content and weight loss in the samples were determined according to [19], [20], respectively. The quantification of carotenoids and provitamin A in the samples was determined according to [21]. The concentration of carotenoid pigments (μg/g of bread) was determined with the respective absorbance and extinction coefficient, for β-carotene (450 nm and 2560), α-carotene (444 nm, 2800), β- cryptoxanthin (451 nm, 2460) and lycopene (472 nm, 3540). Provitamin A (retinol equivalent activity, REA) was estimated with the conversion factor of 1 µg of REA = 12 µg of β-carotene and 24 µg of α-carotene and β-cryptoxanthin.
2.4 Determination of kinetic and thermodynamic parameters
In accordance the studies by [18], [22] the thermal degradation of the carotenoids and provitamin A were expressed with a first-order kinetic reaction, as described in (1).
Where, C0 and Ct are the concentration of the molecules of interest at time 0 and t, k is the degradation rate constant (min-1) and t is the thermal processing time. (D, min), (t0.5, min), Q10, and (z, °C) are the decimal reduction time, half-life time, temperature coefficient and thermal resistance coefficient, respectively. The kinetic parameters were estimated using the Arrhenius equation according to [18], [22]. This equation allows us to model the effect of temperature on the degradation rate constant, where k0, Ea, R and T are the frequency factor (min-1), activation energy (kJ/mol), universal gas constant (8.314 J/mol ºK), and absolute temperature in ºK, respectively. The thermodynamic parameters activation enthalpy (ΔH), inactivation free energy (ΔG), and activation entropy (ΔS) were estimated applying the equations described by [16], where, h is Plank's constant (6.6262 x10-34 J/s), and kB is Boltzmann’s constant (1.3806 x 10-23 J/ºK).
2.5 Statistical analysis
Each of the experiments was carried out in triplicate and the results were expressed as mean values. One-way ANOVA (p < 0.05) was used to study the effect of temperature on the phytochemicals of interest, and the degradation of these molecules was modeled using analytical regression. The analyzes were carried out in SPSS for Windows v.25. and Excel software (version 10, Microsoft Corporation, Redmond, WA, USA). The correlation coefficient R2 was calculated to determine the goodness of fit.
3. RESULTS AND DISCUSSION
3.1 Bread temperature profile during baking
The temperature profiles of the crumb and crust when baking the bread at 160 °C, 180 °C and 200 °C are shown in Figure 1. The temperature increases in the bread crumb followed a sigmoidal pattern, increasing gradually until it became constant at 100 °C. These results agree with [23], who observed a sigmoidal pattern with an inflection point of around 50 °C and a gradual increase in temperature until reaching a constant temperature of 100 °C in the bread crumb. This phenomenon can be explained by the fact that a homogeneous crumb formed by a complete gelatinization of starch is a poor thermal conductor and the phenomenon of evaporation-condensation of water generated in the center of the product [23].
At the beginning, the temperature of the crust was less than 100 °C; at this point, no vaporization occurred through boiling. The production of water vapor in the pores through evaporation was negligible. The temperature increased from the flow of incoming heat in the second baking phase, when the temperature of the crust was higher than 100 °C. At this point, the boiling temperature was reached, and the matter entered the boiling regime. The liquid water turned into steam until exhausted [23]. Once the liquid water was completely evaporated, the temperature of the crust began to rise, approaching the temperature of the oven [23]. This explains the behavior of the temperature profile of the bread crust, where most of the energy supplied in the initial baking stage was used to increase the temperature of the crust, reaching temperatures greater than 100°C in the first 10 min. Subsequently, there was a gradual increase in temperature; at this point, the thermal energy was used to move the evaporation front into the mass and form the crust.
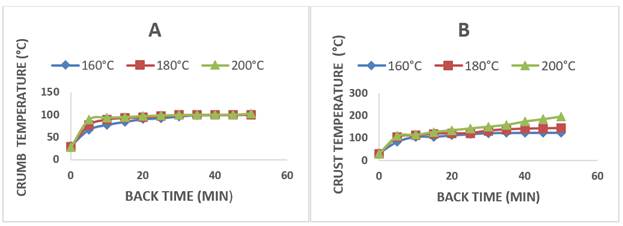
3.2 Loss of weight and moisture during bread baking
Figure 2 shows the loss of weight and moisture in the bread during the baking process. At the end of the process, the samples had a weight loss of 15.11 ± 1.55, 18.49 ± 0.16 and 23.14 ± 0.19 and a moisture loss of 12.60 ± 0.17, 10.87 ± 0.15 and 9.26 ± 0.06 % with baking temperatures of 160 °C, 180 °C, and 200 °C, respectively. These results agree with the studies by Lara et al. [20], [23], who reported a reduction in these two variables when making bakery products and reported the greatest losses of these parameters in the crust. The phenomenon of mass transfer in the crust occurs because of the exposure of the surface to the high temperatures of hot air and to a partial pressure of water vapor in the surrounding air, far from saturation, allowing the diffusion of water vapor in the air [23]. On the other hand, when baking the crumb, there are two gradients that allow water to be retained in the center of the product: one is the concentration gradient, which drives water from the inside out, and the other is the temperature gradient, which drives water vapor to the center. As the temperature in the crumb lowers, the water vapor condenses [23].
3.3 Thermal kinetics and thermodynamic analysis of carotenoids and provitamin A in bread
The initial concentration of carotenoids in the raw dough enriched with mandarin epicarp extract (20 g of extract/100 g of dough) included β-carotene (2.88 µg/g), α-carotene (2.74 µg/g), β-cryptoxanthin (1.70 µg/g), lycopene (0.73 µg/g), and provitamin A (425.24 µg RAE/g). The carotenoid pigments and provitamin A were significantly reduced (p < 0.05) as the bread baking time and temperature increased. These results are consistent with those recorded in other studies, with a degradation of carotenoids after baking processes [7], [24], [25] report losses of carotenoids in baked products such as muffins (48-53 %) and bread (47-67 %). [24] state that the loss of carotenoids during the baking process is associated with the isomerization and hydroxylation of carotenes at high temperatures. The loss of provitamin A after thermal treatments has also been reported by [18], [26] in milk, and tree tomato juice, respectively. [27] report losses of provitamin A between 40-82 % in sweet potatoes after baking, and Muntean [28] reports degradation of provitamin A in bread enriched with pumpkin flour after baking, the researcher comments that this thermal reduction is associated with the degradation of β-carotene, the main carotenoid with provitamin A properties present in the study samples.
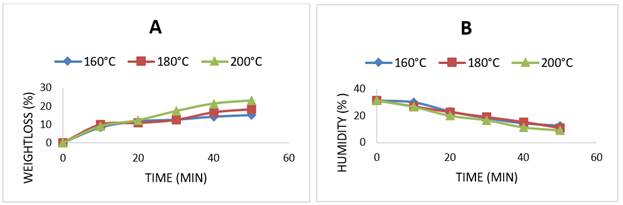
Figure 3 and Table 1 show that, when increasing the intensity of the baking treatment, the constant degradation rate of carotenoids and provitamin A in bread increased, and the correlation coefficient for all kinetics was R2> 0.83. The first-order kinetics of degradation explained the changes in the concentration of carotenoid pigments and provitamin A in the bread after baking. The other kinetic parameters of bread during baking are listed in Table 1. The kinetic parameters D, t0.5, Q10 and z validate that carotenoids and provitamin A are thermolabile compounds during bread baking, presenting different levels of resistance between molecules. The thermal resistance value (z) reports that β-cryptoxanthin presented the highest resistance, followed by lycopene, α-carotene, provitamin A, and β-carotene in the temperature range 160-200ºC (Table 1).
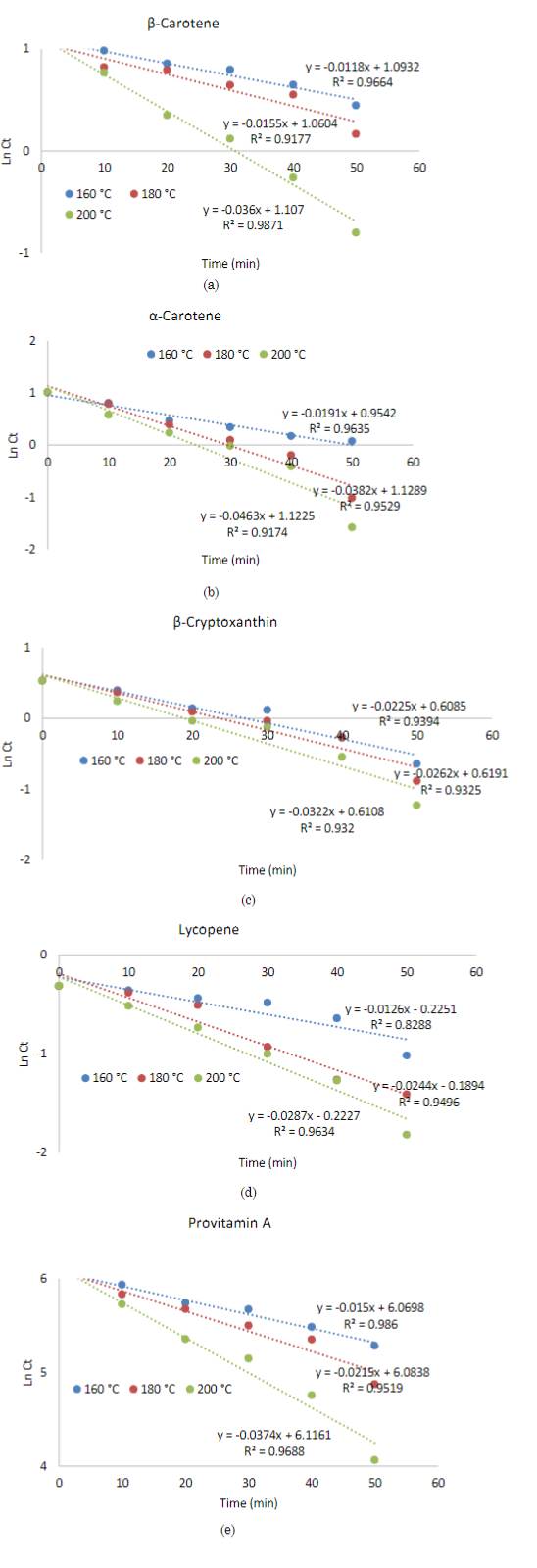
Source: Created by the authors
Figure 3 Thermal degradation kinetic of carotenoids and provitamin A in bread. β-Carotene (a), α- Carotene (b), β-Cryptoxanthin (c), Lycopene (d), and Provitamin A (e)
Table 1 Kinetic parameters in the thermal degradation of carotenoids in bread (160-200 °C)
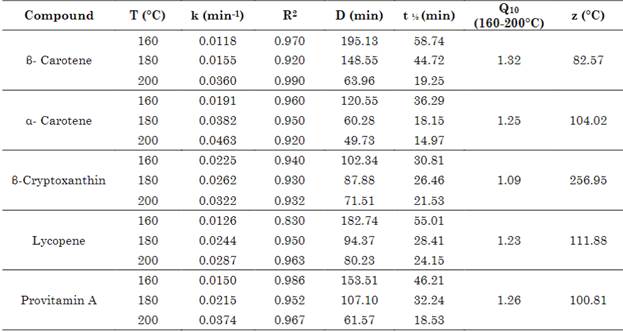
Source: Created by the authors.
To date, research on the thermal degradation of carotenoids and provitamin A in bread during baking processes has not been reported in the scientific literature; however, other studies have applied the hot air dehydration method in different matrices: [29] for Jackfruit fruits (50-70 ºC); and [30] for peaches (50-70 ºC). These researchers reported that first-order kinetics can model changes in carotenoids during thermal treatments in each of the evaluated plant matrices. The degradation of carotenoids during thermal treatments was mainly due to the conjugated carbon double bond structures present in the carotenoids, which make them very sensitive to the action of heat, triggering processes of geometric isomerization, oxidation, and degradation during thermal processing [31].
The thermal sensitivity of β-carotene in this study has also been reported by [32] and [18]. These authors reported that β-carotene is more unstable to heat because of the presence of two rings in its structure and its high sensitivity to isomerization processes. For the thermal stability of β-cryptoxanthin, [33] offered a possible explanation, reporting that this pigment is more stable in thermal treatments because it has hydroxyl groups in its structure that replace conjugated carbon double bonds, providing greater stability against the action of free radicals generated during thermal treatments.
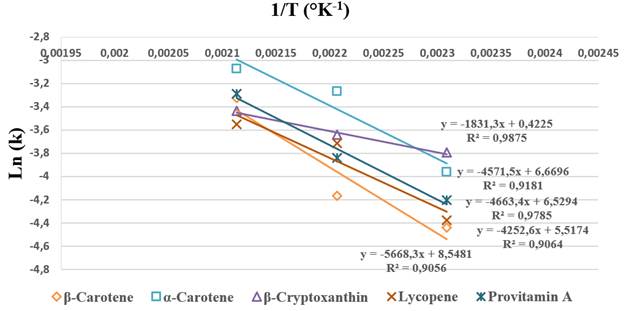
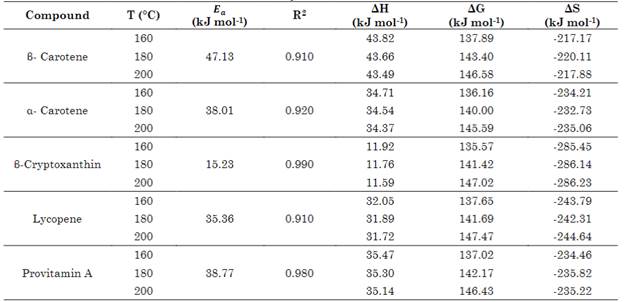
Figure 4 shows the effect of temperature on the constant degradation rate of the carotenoids and provitamin A during the bread baking process in the range of 160 to 200 ºC, which conformed to the Arrhenius model (R2 > 0.91). The activation energy (Ea) during the bread baking process presented the highest value in β-Carotene, followed by provitamin A, α-carotene, lycopene and β-cryptoxanthin (Table 2). Authors such as [32] have indicated that high Ea values represent greater sensitivity in the compound of interest to temperature. [30] estimated Ea in β-cryptoxanthin (120.61 kJ/mol) and β-Carotene (90.85 kJ/mol) during peach dehydration in a temperature range of 50-70 ºC. [34] reported Ea in β-Carotene (89.81 kJ/mol) and lycopene (79.66 kJ/mol) during the thermal treatment of tomato epicarp in vegetable oil in a temperature range of 100-145 ºC.
Source: Created by the authors
Figure 4 Arrhenius plot for degradation of carotenoids and provitamin A in bread
Table 2 Thermodynamic parameters in the thermal degradation of carotenoids in bread (160-200 ° C)
Source: Created by the authors.
For provitamin A, [26] reported Ea values (52.26 kJ/mol) in cow's milk for a temperature range of 70-90 ºC, and Ordoñez-Santos and Martínez-Girón [18] reported Ea values (51.67 kJ/mol) in tree tomato juice in a temperature range of 70-90 ºC. The obvious differences with our results may have resulted from factors such as the type of plant matrix, moisture percentage, and process type. [35] reported that the type of plant matrix affects the cellular structure of carotenoids since these pigments are linked to different proteins that form lipid droplets or crystalline structures that release or retain carotenoids. [30] indicated that the stability of carotenoids in food matrices may be affected by the presence of other bioactive compounds, such as tocopherols, tocotrienols, and vitamin C.
The values of the activation enthalpy (ΔH), inactivation free energy (ΔG), and activation entropy (ΔS) in the degradation of the carotenoids and provitamin A during the baking process at each of the evaluated temperatures can be observed in Table 2. The positive values of ΔH and ΔG and the negative results of ΔS during the bread baking showed that the degradation of the carotenoids and provitamin A was an endothermic reaction that was spontaneous in nature. The studies carried out by [33], and [18] confirmed our results in each of the thermodynamic parameters evaluated during the thermal degradation of carotenoid pigments and provitamin A in the bread baking process. When correlating the values of (ΔS) and (ΔH), a correlation coefficient of R2 = 0.9947 was obtained, indicating a linear compensation between the two thermodynamic parameters evaluated in the thermal degradation of carotenoids during the bread baking process. Other studies carried out by [36], [37], and [18] that evaluated the thermal degradation of carotenoids confirmed the correlation between these two thermodynamic parameters. Reports published by [38], [39], [33], and [18] described how temperature plays an important role in the isomerization and oxidation of carotenoid pigments, forming epoxycarotenoids, apocarotenoids, and low molecular weight structures.
4. CONCLUSIONS
This study showed that the kinetic and thermodynamic model predicted changes in the carotenoids and provitamin A during the bread baking process (160 to 200 ºC). The thermal degradation kinetics followed a first-order reaction with a correlation coefficient of R2 > 0.83 in all bioactive compounds evaluated in the bread. The constant degradation rate of the carotenoids and provitamin A during the bread baking (160 to 200 ºC) was adjusted to the Arrhenius model (R2 > 0.91). The parameters ∆H, ∆G, and ∆S demonstrated that the carotenoid degradation relationship followed an endothermic, non-spontaneous reaction and had less structural freedom than the reactants. These results are important for the design and optimization of bread baking processes since they will maximize the levels of bioactive compounds in this food type.