Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Cancerología
Print version ISSN 0123-9015
rev.colomb.cancerol. vol.17 no.3 Bogotá July/Sep. 2013
Original
Evaluation of the immune response to human papillomavirus types 16, 18, 31, 45 and 58 in a group of Colombian women vaccinated with the quadrivalent vaccine
Evaluación de la respuesta inmune hacia el virus del papiloma humano tipos 16, 18, 31, 45 y 58 en un grupo de mujeres colombianas que recibieron la vacuna tetravalente
Alba Cómbitaa,b,*, Diego Duartea, Josefa Rodrígueza,c, Mónica Molanoa, Lina Martíneza, Pilar Romeroa, Lina Trujillod, Mauricio Gonzálezd, Joaquín Lunad, Natascha Ortize, Gustavo Hernándezf, Pierre Coursagetg, Antoine Touzég
a Grupo de Investigación en Biología del Cáncer, Instituto Nacional de Cancerología (INC), Bogotá, D. C., Colombia
b Departamento de Microbiología, Facultad de Medicina, Universidad Nacional de Colombia, Bogotá, D. C., Colombia
c Unidad de Inmunología, Facultad de Medicina, Universidad del Rosario, Bogotá, D. C., Colombia
d Grupo de Ginecología, INC, Bogotá, D. C., Colombia
e Grupo de Investigaciones clínicas, INC, Bogotá, D. C., Colombia
f Grupo de Investigación en Epidemiología, INC, Bogotá, D. C., Colombia
g Université François-Rabelais, UMR INRA 1282, Tours, France
* Corresponding author. E-mail: acombita@cancer.gov.co (A.L. Cómbita).
Received 21 December 2012; accepted 2 July 2013
Abstract
Objective: To analyze whether the immune response to HPV-16, -18, -31, -45 and -58 capsids in women vaccinated with the quadrivalent vaccine induces cross-reactivity against other HPV virus-like particles (VLPs).
Methods: A total of 88 women aged between 18 and 27 years attending the HPV clinic at the Instituto Nacional de Cancerología were enrolled and vaccinated against HPV. Follow-up visits were scheduled at months 7, 12, and 24. Samples were collected for cytology, HPV-DNA typing, and detection of HPV antibodies. IgG antibodies were measured by ELISA using HPV-16, -18, -31, -45, and -58 VLPs. HPV-DNA detection was done by GP5+/GP6+PCR-ELISA and HPV typing was performed by Reverse Line-Blot assay.
Results: Pre-vaccination, the seroprevalence of HPV-16, -18, -31, -45, and -58 was 39%, 31.7%, 15.9%, 31.7%, and 23.2%, respectively. One month post-vaccination, the seroprevalence increased close to 100% for all types. At month 24, this response was maintained only for HPV-16 and -18. For HPV-31, -45 and -58, the seroprevalence decreased to below 50%. The prevalence of HPV DNA types 16, 18 and 58 before vaccination was little changed 1 month after vaccination. No new infections were observed at 24 months. For HPV-16 and -18 related types, no differences were observed before vaccination and at month 24. For other high-risk HPV types, the prevalence increased 18 months post-vaccination (15.5%) compared with pre-vaccination (9.8%).
Conclusion: Immune response to all HPV types increased after vaccination, but this increase was maintained only for HPV-16 and -18. These results suggest a possible cross-reactivity against HPV types 31, 45 and 58, but this cross-reactivity wanes with time. © 2012 Instituto Nacional de Cancerología. Publicado por Elsevier España, S.L. Todos los derechos reservados.
Keywords: Papillomavirus Vaccines; Immunity; Seroprevalence.
Resumen
Objetivo: Analizar si la respuesta inmune hacia las cápsides del VPH tipos 16, 18, 31, 45 y 58 en mujeres que recibieron la vacuna tetravalente induce reactividad cruzada hacia otros tipos virales.
Métodos: Ochenta y ocho mujeres entre 18 y 27 años, asistentes al Grupo VPH del Instituto Nacional de Cancerología, recibieron la vacuna de VPH. Visitas de seguimiento en los meses 7, 12 y 24. Se tomaron muestras para prueba de Papanicolaou, tipificación de VPH y detección de anticuerpos. Los anticuerpos se detectaron por ELISA, usando VLP-VPH. La detección del ADN-VPH se realizó por Reverse Line Blot.
Resultados: Prevacunación, la seroprevalencia de VPH tipos 16, 18, 31, 45 y 58 fue de 39, 31,7, 15,9, 31,7 y 23,2%, respectivamente. Al mes 7 aumentó cerca del 100% para todos los tipos. Al mes 24 esta respuesta se mantuvo para VPH tipos 16 y 18. Para VPH tipos 31, 45 y 58 disminuyó por debajo del 50%. La prevalencia de ADN-VPH tipos 16, 18 y 58 tuvo poca variación antes y un mes después de la vacunación. Al mes 24, no se observaron nuevas infecciones. Para VPH tipos 16 y 18, no se observaron diferencias antes ni al mes 24. En otros tipos de HR-VPH aumentó la prevalencia al mes 24 (15,5%), comparada con la prevacunación (9,8%).
Conclusión: Se observó un aumento de la respuesta inmune a todos los tipos de VPH después de la vacunación, pero esta se mantuvo solamente para los VPH tipos 16 y 18. Los resultados sugieren una posible reactividad cruzada contra VPH tipos 31, 45 y 58. Sin embargo, esta reactividad cruzada disminuye con el tiempo.
Palabras clave: Vacunas contra Papillomavirus; Inmunidad; Seroprevalencia.
Introduction
The recognition of an etiologic association between high-risk human papillomavirus (HPV) infection and cervical cancer has promoted the development of prophylactic vaccines for the prevention of HPV infection1. To date, two prophylactic HPV vaccines have been developed commercially: CervarixTM, from GlaxoSmithKline, is a bivalent vaccine composed of virus-like particles (VLPs) made from recombinant L1 proteins of HPV-16 and -18 and formulated with an adjuvant ASO4, which contains a combination of aluminum hydroxide and the immunostimulant MPL2 (3-O-desacyl-4′-monophosphoryl lipid A). GardasilTM, from Merck, is a quadrivalent valent vaccine composed of VLPs from HPV-16, -18, -6, and -11 formulated with an aluminum hydroxyphosphate-based adjuvant3.
Numerous clinical trials have shown that both vaccines exhibited high prophylactic efficacy (up to 100%) in preventing incident HPV-16 and -18 infections and their associated precancerous lesions4-12. These vaccines induce high levels of serum IgG antibodies against VLP HPV-16 and -18, which are up to 1000 times higher than those observed after natural infection. The protection afforded by these vaccines has been demonstrated up to 8.4 years post-vaccination for the HPV 16/18 vaccine13 and 5 years post-vaccination for the HPV 6/11/16/18 vaccine11.
It is recognized that the protective efficacy of HPV vaccines is mediated by anti-L1 humoral response14,15. These antibodies are predominantly type-specific; however, a weak cross-reactivity has been reported between very closely related types such as HPV 6/11, HPV 18/45 and HPV 16/3116-20 . Several clinical studies indicate that HPV vaccines may confer protection against some phylogenetically-related HPV types but not against distantly-related types10,21. It has been reported that the bivalent (HPV16/HPV18) vaccine induces protection against incident infections and premalignant lesions with HPV-45 (HPV-18-related type) and HPV-3122-24 (HPV 16-related type). In contrast, for the quadrivalent HPV vaccine, cross-protection against HPV-45 has been reported, but not against CIN1-3 or adenocarcinomas in situ associated with this type (19) Moreover, a modest reduction in HPV-31, -33, -45, -52, -58-related CIN2, CIN3 and AIS has been observed. These findings suggest that vaccination could induce a protective immune response against HPV types not included in the vaccine.
Previous epidemiological studies have shown geographic differences in the prevalence of HPV. In these studies, although HPV16 was the predominant type detected, marked differences were noted in the prevalence of other high-risk HPV types25-27. In Colombia, a study of the prevalence and determinants of HPV infection among Colombian women with normal cytology showed wide diversity of HPV infections. Up to 32 different HPV types were detected in women with normal cytology. In that study, HPV-16 followed by -58, -56, -52, -81, -51 and -18 were the most prevalent HPV types detected as single infections in all age groups28. Due to this difference in HPV prevalence in our population, it is important to determine whether the immune response generated against HPV-16 and HPV-18 induces cross-reactivity against other HPV types frequently detected in Colombia. The objective of this study was to analyze whether the humoral immune response to HPV-16, -18, -31, -45 and -58 viral capsids in a group of women vaccinated with the quadrivalent vaccine induces cross-reactivity against other HPV VLPs that are common in our population.
Methods
Study population
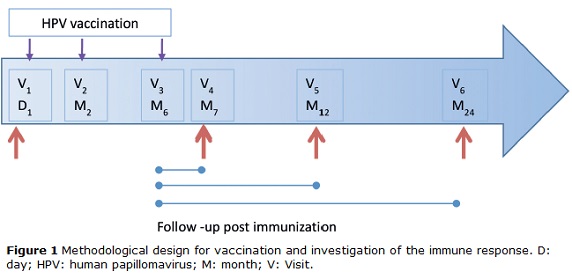
This is a descriptive study, which was conducted in 95 women from the population of Bogotá, Colombia. Of these, 29 had formed part of the placebo group of the Future I study and were vaccinated when blinding was removed. All participants were enrolled at the HPV Clinic of the National Cancer Institute, Bogota Colombia from September 2007 to July 2008 and the participants were followed up for 2 years. Women were eligible to participate if they (a) were aged between 18 and 27 years, (b) were permanent residents of Bogotá, (c) were not currently pregnant and had no intention of becoming pregnant during the first year of follow-up, and (d) had not reported any concomitant immunosuppressive diseases. Of 95 women, seven were excluded because they were ineligible. A total of 88 participants received the complete vaccination scheme; however, six were excluded because they did not return to follow-up visits. All participants underwent a pregnancy test prior to each injection. The vaccine schedule consisted of three injections given on day 1 and at moths 2 and 6. The follow-up consisted of three visits at months 7, 12 and 24 (with a ±1 month visit window, except for month 7, which had a window of ±15 days) (Fig. 1). Cervical specimens were taken for cytological assessment and HPV-DNA detection at day 1 and at each follow-up visit. Similarly, blood samples were taken to measure the serologic HPV response at the same schedule. The samples were processed and stored at −70°C until use. At each follow-up visit, participants completed a questionnaire designed to collect information on lifestyle, sexual behavior, contraceptive use, and sociodemographic and reproductive characteristics. This study was approved by the INC Medical Ethics Committee. Informed consent was obtained from all participants.
HPV-DNA detection by polymerase chain reaction and Typing by an enzyme immunoassay based assay.
HPV-DNA detection was performed by a standard GP5+/GP6+ polymerase chain reaction (PCR) and enzyme immunoassay (EIA)-based assay29,30. Briefly, samples were subjected to EIA HPV DNA group-specific analysis using a cocktail of probes for 37 high- and low-risk HPVs. HPV-positive samples were then assessed by Southern blot hybridization of GP5+/ GP6+ PCR products with general probe of specific DNA fragments. Samples that were positive by EIA-based assay but negative by Southern blot assay were considered as being of undetermined type and were classified as low risk. This PCR-EIA was also used as a semi-quantitative method to assess the relative amount of HPV-DNA in cervical scrapes.
HPV typing was performed by the Reverse Line Blot technique, following the methodology described by van den Brule et al.31. This technique allows the characterization of 37 different viral types. Briefly, amino-labeled probes specific for each of the viral types were covalently bound to a nitrocellulose membrane (Byodine C) positively charged with EDAC reagent (Sigma, St. Louis, MO, US) in parallel lines through the use of a miniblotter. The sites where the probe failed to bind were blocked with a 100-mM NaOH solution. Subsequently, the membrane was rotated 90° in a miniblotter and the biotin-labeled GP5+/GP6+ PCR products for each sample were added. Consequently, each PCR product was in contact with the 37 probes attached to the membrane. Hybridization was performed at 42°C for 45 min and after two washes, the peroxidase-labeled streptavidin was added. Finally, hybridization was revealed using the SL chemiluminescence kit (Amersham, Buckinghamshire, UK) according to the manufacturer›s instructions.
Production of HPV-VLP and detection of anti-HPV antibodies by ELISA
HPV type-specific VLPs were produced in SF21 insect cells using recombinant baculoviruses encoding HPV-16, -18, -31, -45 and -58 L1 proteins and were purified as previously described32. The ELISA was performed as previously described with some modifications32. 96-Well micro plates (Nucn, Sigma-Aldrich, St. Louis, MO, US) were coated overnight at 4 °C with 200 ng of each HPVL1 VLPs or 200 ng bovine serum albumin (BSA) in phosphate-buffered serum (PBS). Each serum sample and BSA was tested twice. The four samples from each follow-up visit were analyzed on the same plate. After washing with PBS, 200 ml of PBS containing 5% fetal bovine serum (FBS; GIBCO, life technologies, Grand Island, NY, US) and 0.1% Tween 20 were added for 2 h at 37°C. The blocking solution was replaced by 100 ml of sera diluted 1:20 in 5X PBS - 10% FBS and 2% Tween-20. Plates were incubated at 37 °C for 60 min. After five washes, bound antibodies were detected with a horseradish peroxidase conjugated polyclonal rabbit anti-human IgG immunoglobulin diluted 1:5000 (Vector Laboratorios, Inc). Following incubation at 37°C for 1 h and four washes, 100 ml of a substrate solution containing 0.1% 2,2′-azino-bis-3-ethylbenz-thiazoline-6-sulfonic-acid (ABTS; Sigma-Aldrich, St. Louis, MO, US) in 50 mм citrate/phosphate buffer and 0.03% hydrogen peroxide were added. Optical density (OD) values were determined at 405 nm with an automated plate reader (Labsystems Multiskan® MCC/340, Ani Labsystems Ltd. Oy. Tiilitie 3, Finland). The background reactivity observed in the BSA-coated wells was subtracted from the OD found in each of the HPV VLP-coated wells for each sample.
The cut-off level above which OD values were considered positive was based on the distribution of OD values observed from 70 serum samples with normal cytology negative for HPV-DNA and anti-HPV IgG. This value was calculated independently for each VLP type. We used an iterative statistical approach that excluded outliers in the distribution of test results until no remaining value was greater than three times standard deviations above the mean OD of the serum samples of the women included. The cut-off values for IgG anti-HPV-16, -18, -31, -45 and -58 were 0.216, 0.247, 0.254, 0.340, and 0.287 respectively. The OD values above this cut-off were subsequently considered as seropositive.
Statistical analysis
Serology results were dichotomized as positive or negative according to the cut-off. The prevalence of HPV-DNA and the seroprevalence were calculated independently for each type at baseline and post-vaccination. The analysis was performed using the Statistical Package for Social Sciences (SPSS) version 19. We used a Chi-square test to evaluate the difference of proportions between day 1 and months 7, 12 and 24 after the first dose for specific types of HPV-DNA and for the prevalence of IgG anti-HPV VLP 16, 18, 31, 45, and 58.
Results
Characteristics of the study population
A total of 95 women from the population of Bogotá (Colombia) were included in this study. Of these 95 women, seven were excluded because they did not meet the inclusion criteria and six were discontinued because they did not attend the follow-up visits. In total, 82 women aged 18 to 27 years were included and were vaccinated and followed-up according the vaccination scheme. Of the 82 women included, 81, 73 and 71 participants attended the fourth, fifth and sixth follow-up visits, respectively, and only 66 women attended all visits. At baseline, the mean age of the participants was 23.6 years (range, 18-27 years). A total of 37.8% of the participants had a high educational level (university), while 35.4% had only a basic education level (high school). More than half (59.8%) of the participants had their first sexual intercourse between the ages of 16 and 19 years and 67.1% had had between two and five sexual partners. Almost all (93.9%) reported using contraception; the most frequently used was the condom (25.9%), followed by the intrauterine device (22.2%) and oral contraceptives (19.8%).
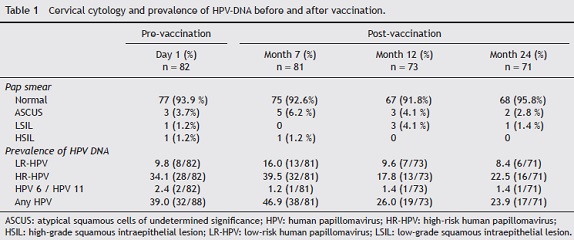
The status of cervical cytology and the prevalence of high risk, low risk and total HPV DNA are described in Table 1. At baseline, the prevalence of HPV-DNA of any type was 39% (32/82). This prevalence increased slightly 1 month after vaccination (46.9%), and decreased to 26% and 23.9% 6 and 18 months after vaccination, respectively.
Seropositivity to HPV-16, -18, -31, -45 and -58 in women vaccinated with the quadrivalent vaccine
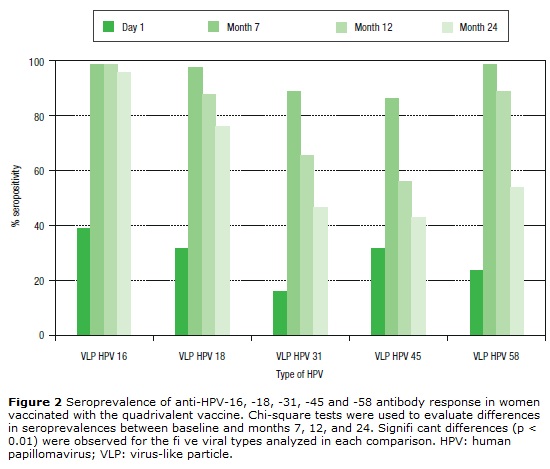
Seropositivity to HPV-16, -18, -31, -45 and -58 in women vaccinated with the quadrivalent vaccine is shown in Figure 2. Before vaccination, seropositivity to HPV-16, -18, -31, -45 and -58 was 39% (32/82), 31.7% (26/82), 15.9% (13/82), 31.7% (26/82), and 23.2% (19/82), respectively. At month 7 after vaccination, a high level of seroconversion was observed for both vaccine and non-vaccine HPV types. The seropositivity rates for anti-HPV-16, -18, and -58 were 98.8% (80/81), 97.5% (79/81), and 98.7 (80/81), respectively. For HPV-31 and -45, the seropositivity rates were 88.8% (72/81) and 86.4% (70/81), respectively. At month 12 after vaccination, seropositivity to VLP HPV -16, -18 and -58 remained high: 98.6% (72/73), 87.7% (64/73), and 89.0% (65/73), respectively, while a marked decrease was observed for HPV-31 and -45: 65.7% (48/73) and 56.2% (41/73), respectively. At month 24, seropositivity to HPV-16 was 95.8% (68/71), while that for HPV-18 was 76% (54/71). For other HPV types not included in the vaccine, seropositivity fell by around 50%. For HPV-31, -45 and -58, seropositivity decreased to 46.5% (33/71), 38% (27/71), and 53.5% (38/71), respectively.
Prevalence of HPV DNA in vaccinated women
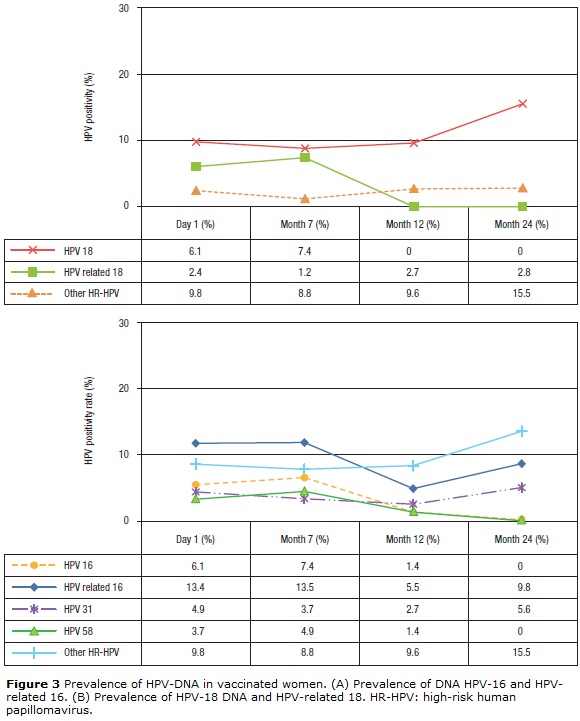
At baseline, the prevalence of HPV-6 and -11 was 2.4% and decreased slightly after vaccination (Table 1). For HPV-16 and -18, the prevalence at baseline was 6.1% for both types and remained similar 1 month after vaccination (7.4%). However, at month 12, the prevalence decreased to 2.7% for HPV 16, while for HPV-18 no infections were observed. At month 24, no HPV-16 or -18 infections were observed (Figs. 3A-B). Concerning HPV-16-related types, the prevalence before vaccination was 13.4% (11/84), remained similar 1 month after vaccination (13.5%, 11/83), and decreased 6 months after vaccination (5.5%). However, at month 24 after vaccination, the prevalence increased to 9.8% (p=0.254) (Fig. 3A). When the frequency of HPV-31 DNA was evaluated specifically, a reduction was observed at months 7 and 12 (3.7%, 3/81 and 2.7%, 2/71 respectively) (p= 0.001) compared with baseline (4.9%, 4/82). However, an increase to 5.6% (4/71) was detected at month 24 (Fisher's exact test, p=0.014). For HPV-58, this frequency was 3.7% (3/81) at baseline and increased slightly to 4.9% (4/81) at month 7 (p=0.103). However, no infections were observed at month 24. For HPV-18-related types, the prevalence was low before and after vaccination (2.4%, 1.2%, 2.7%, and 2.8%) (p>0.05) (Fig. 3B). For HPV-45, only 2.7% (2/73) of the women were positive at month 12 (data not shown). For other unrelated high-risk HPV types, an increase in prevalence to 15.5% was observed at month 24 compared with before vaccination (9.8%) (Figs. 3A-B).
Discussion
An ideal prophylactic vaccine against HPV would induce anti-HPV antibodies broadly reactive for the major oncogenic HPV types. It has been previously shown that the HPV L1 protein is highly immunogenic and conserved. Moreover, the neutralizing antibodies generated in response to vaccination are conformationally dependent and type specific. However, a cross-reactive immune response against heterologous HPV types has been detected (12,23,33). In this study, we analyzed whether the immune response induced in Colombian women vaccinated with the quadrivalent vaccine induces cross-reactivity against other HPV VLPs.
As previously reported33,34, in this study we also observed high anti-HPV-16 and -18 immune responses at month 7 after vaccination (close to 100%). Similarly, anti-HPV-16 antibody seropositivity remained high after 24 months of follow-up, while seropositivity to HPV-18 slightly declined during the follow-up. Only 76% of the women remained seropositive at month 2411,14,33,35. These results are in agreement with those previously reported by Romanowski, who found that while anti-HPV-16 antibodies persisted after immunization with the quadrivalent vaccine, HPV-18 antibodies declined36.
Moreover, in agreement with other studies37,38, no new infections with HPV-16 or -18 occurred in vaccinated women.
Like previously studies, we also observed that the vaccine induced antibodies against non-vaccine oncogenic HPV types10,12,21,24,34,39,40. One month post-vaccination, the seroconversion rate to HPV-31 and -45 increased (between 86.4% and 98.8%). However, at month 24 post-vaccination, seroprevalence markedly declined (46.5% and 38% for HPV-31 and -45, respectively), while Einstein et al. observed a seroprevalence of 69% and 74%, respectively. These differences can be explained by differences in the tests used or in the study population. However, as in other reports, although new HPV-31 infections declined at months 7 and 12 after vaccination compared with baseline, a slight increase was detected at month 24. These results are in agreement with those of previous reports suggesting that cross-protection of the quadrivalent vaccine is more modest40.
In previous studies, no significant increase in HPV-58 neutralization titres23,24 or lower neutralizing antibody titres against HPV-58 were found after vaccination23. In our study, we found an increase in the frequency of antibodies to HPV-58 after vaccination, although this increase was non-significant. However, no new infections were observed at month 24 of follow up. This discrepancy could be partly due to differences in the tests used. In the Draper and Kemp studies, the authors used a pseudovirion-based neutralization assay to analyze the presence of cross-neutralizing antibodies to HPV-58, which measures only neutralizing antibodies, while in this study, we tested antibodies by ELISA, which measures total antibody concentrations and does not distinguish between neutralizing and non-neutralizing types. In addition, a limitation of our study is the short follow-up as well as the small number of participants, suggesting the need to track efficacy over time, as HPV-58 is the second most common type in our population.
In conclusion, an increase in HPV seroprevalence for all types investigated was observed after vaccination. However, high levels of seroprevalence were maintained only for HPV-16 and -18. The data also suggested the possible induction of cross-protection against HPV-31, -45 and -58. However, the rapid decrease in seroprevalence against these types suggested that protection does not last for a long period after immunization.
Financial disclosure
This work was supported by accord Ecos Nord - Colciencias grant No 368-2007 and INC/DNP grant No 41030310-20.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors sincerely thank all individuals who participated in this study for their generosity. We thank the HPV group of the Instituto Nacional de Cancerología, Bogotá, Colombia.
References
1. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-9. [ Links ]
2. Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757-65. [ Links ]
3. Tovar JM, Bazaldua OV. New quadrivalent HPV vaccine developments. Postgrad Med. 2008;120:14-6. [ Links ]
4. Villa L, Pérez G, Kjaer S, et al. Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis. 2007; 196:1438-46. [ Links ]
5. Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-27. [ Links ]
6. Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007;369:1861-8. [ Links ]
7. Garland SM, Hernández-Ávila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-43. [ Links ]
8. Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006;367:1247-55. [ Links ]
9. Joura EA, Leodolter S, Hernández-Ávila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-702. [ Links ]
10. Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009;374:301-14. [ Links ]
11. Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459-66. [ Links ]
12. Wheeler CM, Castellsague X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the rando mised, double-blind PATRICIA trial. Lancet Oncol. 2012; 13:100-10. [ Links ]
13. Roteli-Martins CM, Naud P, De BP, et al. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother. 2012;8:390-7. [ Links ]
14. Joura EA, Kjaer SK, Wheeler CM, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26:6844-51. [ Links ]
15. Suzich JA, Ghim SJ, Palmer-Hill FJ, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553-7. [ Links ]
16. Christensen ND, Reed CA, Cladel NM, Hall K, Leiserowitz GS. Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type-specific epitopes on HPV-6 28. Virology. 1996; 224:477-86. [ Links ]
17. Cómbita AL, Touze A, Bousarghin L, Christensen ND, Coursaget P. Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses 6. J Virol. 2002;76:6480-6. [ Links ]
18. Fleury MJ, Touze A, Álvarez E, et al. Identification of type specific and cross-reactive neutralizing conformational epitopes on the major capsid protein of human papillomavirus type 3. Arch Virol. 2006;151:1511-23. [ Links ]
19. Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin. 2007;3:109-15. [ Links ]
20. Yeager MD, Aste-Amezaga M, Brown DR, et al. Neutralization of human papillomavirus (HPV) pseudovirions: a novel and efficient approach to detect and characterize HPV neutralizing antibodies. Virology. 2000;278:570-7. [ Links ]
21. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199:926-35. [ Links ]
22. Ault KA. Human papillomavirus vaccines and the potential for cross-protection between related HPV types. Gynecol Oncol. 2007;107:S31-S33. [ Links ]
23. Draper E, Bissett SL, Howell-Jones R, et al. Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera. Vaccine. 2011;29:8585-90. [ Links ]
24. Kemp TJ, Hildesheim A, Safaeian M, et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011;29:2011-4. [ Links ]
25. Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991-8. [ Links ]
26. Sanjosé de S, Díaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a metaanalysis. Lancet Infect Dis. 2007;7:453-9. [ Links ]
27. Lazcano-Ponce E, Herrero R, Muñoz N, et al. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer. 2001;91:412-20. [ Links ]
28. Molano M, Posso H, Weiderpass E, et al. Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer. 2002;87:324-33. [ Links ]
29. de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3' ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76(Pt 4):1057-62. [ Links ]
30. Molano M, Van den Brule A, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158:486-94. [ Links ]
31. van den Brule AJ, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJ, Snijders PJ. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol. 2002;40:779-87. [ Links ]
32. Cómbita AL, Bravo MM, Touze A, Orozco O, Coursaget P. Serologic response to human oncogenic papillomavirus types 16, 18, 31, 33, 39, 58 and 59 virus-like particles in colombian women with invasive cervical cancer. Int J Cancer. 2002;97: 796-803. [ Links ]
33. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5:705-19. [ Links ]
34. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum Vaccin. 2011;7:1359-73. [ Links ]
35. Einstein MH, Baron M, Levin MJ, et al. Comparative immuno genicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum Vaccin. 2011;7:1343-58. [ Links ]
36. Romanowski B. Long term protection against cervical infection with the human papillomavirus: review of currently available vaccines. Hum Vaccin. 2011;7:161-9. [ Links ]
37. Romanowski B, de Borba PC, Naud PSm et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374: 1975-85. [ Links ]
38. Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26 (Suppl 10): K53-K61. [ Links ]
39. Kemp TJ, Safaeian M, Hildesheim A, et al. Kinetic and HPV infection effects on cross-type neutralizing antibody and avidity responses induced by Cervarix®. Vaccine. 2012;31: 165-70. [ Links ]
40. Wheeler CM, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J Infect Dis. 2009;199:936-44. [ Links ]