Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous group of neoplasms that have in common certain morphological, immunophenotypic, and genetic characteristics. DLBCL makes up from 25% to 40% of all non-Hodgkin lymphomas (NHL), and is the most common histological subtype worldwide. In Ecuador, it accounted for 49% of all NHL cases from 2006 to 20101-3.
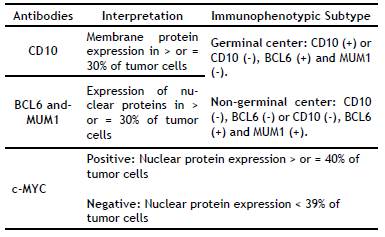
In 2008, the World Health Organization (WHO) put forward a new DLBCL classification that includes morphological and molecular variants and immunophenotypic subgroups, resulting in two subtypes: germinal center (GC) and non-germinal center (NGC). This division is based on the Hans immunohistochemical algorithm (CD10, BCL6, and MUM1), which has a high reproducibility and concordance (80-96%) with gene expression profiling using DNA microarrays4-8.
The results of scientific studies on the immunophenotypic division of DLBCL have been published in Europe, Asia, Latin America (Colombia, Brazil, and Guatemala), and the United States. Despite its recognized prognostic and therapeutic value, the immunophenotypic profile of DLBCL in Ecuador is unknown. This study is the first immunophenotypic classification of DLBCL and its correlation with the overexpression of c-MYC protein in the Ecuadorian population8-14.
Materials and Methods
Patients: We compiled all the DLBCL cases between January 2006 and December 2015 (n = 38) at the Pathology Service of the Metropolitan Hospital in Quito, Ecuador. We excluded 11 cases because they contained insufficient histopathological material to perform the required analyses, ending up with a final sample of 27 cases.
Histopathology: The selected paraffin blocks were surgical specimens and biopsies processed according to the Service's protocols. DLBCL cases were selected if the paraffin blocks contained adequate tissue for manual tissue microarray construction. The DLBCL diagnoses were based on the current 2008 WHO criteria for classification of tumors of hematopoietic and lymphoid tissues.
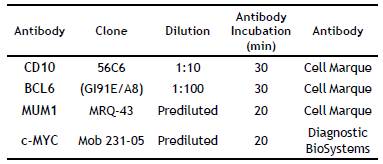
Manual Tissue Microarrays and Immunohistochemistry: The best-preserved area with appropriate morphology was selected to construct manual tissue microarrays with a diameter of 0.4 cm. Sections with a thickness of 4(jm were cut and then stained with hematoxylin-eosin (H&E); then immunohistochemical techniques for CD10, BCL6, MUM1, and c-MYC (Table 1 and 2).
Statistical Analysis: The epidemiological design was descriptive as a whole regarding all the samples from DLBCL-diagnosed patients in whom the frequency of the immunophenotypic subtypes GC and NGC was determined. The qualitative variables were expressed in simple frequencies and percentages, and the quantitative variables were expressed in averages and standard deviation. Moreover, the expression of the c-myc protein expression was also investigated for each immunophenotypic subtype of DLBCL.
Results
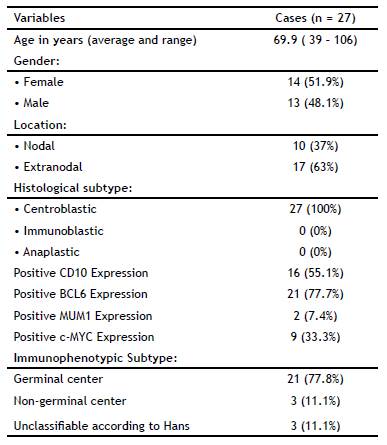
Table 3 summarizes the patients' clinical-pathological characteristics. The average age of the 27 patients was 69.9 ± 16.9 years (range: 39 to 106 years); 51.9% of the subjects (n = 14) were female. The average age of the studied women was 70.5 ± 16.8 years; it was 69.2 ± 17.6 years (p > 0.05) for men. The most frequent locations were extra-nodal (n = 10), predominantly the colon and stomach. H&E staining showed that all the cases had a centroblastic morphology. The immunophenotypic subtype was established according to Hans algorithm. The GC subtype accounted for 77.8% of cases; 11.1% of cases were NGC and 11.1% were unclassifiable. The GC subtype predominantly overexpressed c-MYC (in 40.7% of cases).
Discussion
Classification of DLBCL in GC and NGC subtypes has been carried out in a number of studies worldwide. In Latin America (Guatemala, Brazil, and Colombia), very little has been published on this subject 7,8,9,10. In Ecuador, our results show GC to be the most frequent immunophenotypic subtype of DLBCL (42.9% versus 33.3% NGC). These results are similar to those obtained by Perry in Guatemala, where the GC subtype predominated. In Colombia, Ospina found GC to be the most frequent subtype (n = 61), whereas Castellanos reported an NGC predominance (n = 40)1, 2,9,10. It is worth noting that two studies in Latin American countries with very different population figures -Brazil, with 210,616,910 inhabitants, and Ecuador, with 16,507,400- were based on the same number of cases (n = 27), but they show a different immunophenotype, with predominance of the NGC subtype in Brazil and the GC in Ecuador8,11-14.
Studies published in Asian countries such as Korea (n = 381), Taiwan (n = 153) and China (n = 114) used Hans algorithm; they concluded that the most common subtype was NGC9,10.
The international multi-institutional study "Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray" compiled data from 152 patients at different universities and cancer centers [Nebraska Lymphoma Study Group Registry (n = 49); British Columbia Cancer Center (n = 30); University of Würzburg (n = 29); Norwegian Radium Hospital (n = 29); University of Barcelona (n = 8); and the Southwest Oncology Group (n = 7)]. This study concluded that NGC was the most frequent immunophenotype (n = 88). Ten cases could not be classified according to Hans algorithm, requiring the use of other algorithms, such as Choi et al.8,15
The WHO recommends Hans algorithm for DLBCL classification because of its high reproducibility and concordance (80-96%) with gene expression profiling through DNA microarrays. Nevertheless, some cases cannot be classified through this algorithm, and organizations such as the College of American Pathologists recommend other algorithms, such as Choi's (MUM-1, CD10, BCL6, and FOXP1) or Tally's (MUM-1, CD10, FOXP1, and LMO2) in such cases3. In our study (n = 27), three cases were unclassifiable according to Hans algorithm. Castellanos (n = 103) reported six such cases in Colombia, and, as previously mentioned, the international multi-institutional study of 152 patients reported ten of such cases. These figures indicate that the majority of DLBCL cases are properly classified by the Hans algorithm, and a minimal number of cases require the application of other algorithms8,12.
Our study identified c-MYC overexpression in 40.7% (95% CI: 22.1 to 59.2%) of cases with GC subtype predominance (42.9% of cases). These results are analogous to those of Gupta et al. (n = 40), in which 50% of cases overexpressed c-MYC by IHC and 65% were of the GC subtype. They are also similar to the results of the Perry study (n = 106), in which 46% of patients who overexpressed c-MYC were of the GC subtype. Johnson et al. (n = 72) and Kluk M et al. (n = 77) reported overexpression of c-MYC by IHC in 33°% and 11.5°% of their DLBCL cases, respectively14,16-18.
This study is the first immunophenotypic classification of DLBCL with the overexpression of c-MYC protein in the Ecuadorian population. The GC subtype accounted for 77.8% of cases; 11.1% of cases were NGC and 11.1% were unclassifiable. The GC subtype predominantly overexpressed c-MYC (in 40.7% of cases).
Currently, overexpression of the c-MYC protein is considered an IPI-independent prognostic factor for both disease-free and overall survival. Patients who overexpress the c-MYC protein have larger tumors, higher chemo-resistance, a worse prognosis, and shorter survival19-25.
It is required to complement the amplification study of the myc gene by fluorescent in situ hybridization (FISH).