Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Colombiana de Psicología
Print version ISSN 0123-9155
Act.Colom.Psicol. vol.17 no.2 Bogotá July/Dec. 2014
https://doi.org/10.14718/ACP.2014.17.2.7
ARTÍCULO
http://dx.doi.org/10.14718/ACP.2014.17.2.7
EFFECTS OF CB1 CANNABINOID RECEPTOR ACTIVATION IN THE NUCLEUS ACCUMBENS SHELL ON FEEDING BEHAVIOR
EFECTOS DE LA ACTIVACIÓN DEL RECEPTOR CANNABINOIDE CB1 EN EL NÚCLEO ACCUMBENS SHELL SOBRE LA CONDUCTA ALIMENTARIA
EFEITOS DA ATIVAÇÃO DO RECEPTOR CANNABINÓIDE CB1 NO NÚCLEO ACCUMBENS SHELL SOBRE A CONDUTA ALIMENTAR
FELIPE CORTÉS-SALAZARa, JOSUÉ OMAR SUÁREZ ORTÍZa, NANCY MÓNICA CENDEJAS TREJOa, JUAN MANUEL MANCILLA-DÍAZa, VERÓNICA ELSA LÓPEZ-ALONSOa, RODRIGO ERICK ESCARTÍN-PÉREZa*
a Neurobiology of Eating Laboratory, Universidad Nacional Autónoma de México, Fes Iztacala. Tlalnepantla, México
* R. Erick Escartín-Pérez. Neurobiology of Eating Laboratory, UNAM FES Iztacala. 1 Av. de los Barrios, Los Reyes Iztacala, Tlalnepantla, México, 54090, México. Tel: + (52 55) 56231333 ext 39717. FAX: + (52 55) 5390 7604. escartin@campus.iztacala.unam.mx. Acknowledgements. Funding for this study was provided by UNAM DGAPA grant IN224811 and IN224214 and FESI PAPCA 15.
Referencia: Cortés-Salazar, F., Suárez Ortíz, J. O., Cendejas Trejo, N. M., Mancilla-Díaz, J. M., López-Alonso, V. E. & Escartín-Pérez, R. E. (2014). Effects of CB1 cannabinoid receptor activation in the nucleus accumbens shell on feeding behavior. Acta Colombiana de Psicología, 17(2): pp. 61-68. DOI: 10.14718/ACP.2014.17.2.7
Recibido, mayo 2/2014
Concepto evaluación, mayo 12/2014
Aceptado, junio 23/2014
Abstract
Obesity and its related pathologies are well- known health hazards. Although obesity and overweight have multifactorial causes, overeating is common in both of these conditions. According to animal models, endocannabinoids and their receptors in the brain play a key role in the genesis and development of obesity. It has been proposed that the cannabinoid receptors CB1 (RCB1) expressed in the nucleus accumbens shell (NAC) are involved in the increase of the hedonic properties of food. To test this hypothesis, this study aimed to assess the effects of activating the NACs RCB1 on standard food intake during the light phase of the light-dark cycle. The effects of activating the RCB1 with CP 55,940 and WIN 55-212-2 (0.125, 0.25 and 0.5 nmol) in the NACS on feeding behavior and the behavioral satiety sequence of rats were assessed. It was found that both agonists increased food intake and delayed expression of satiety during the light phase. These results suggest that cannabinoid agonists encourage food intake when motivation is low and palatability is normal.
Key words: Cannabinoids, food, nucleus accumbens shell, behavioral satiety sequence.
Resumen
La obesidad y sus patologías relacionadas son riesgos de salud muy conocidos. Aunque la obesidad y el sobrepeso tienen causas multifactoriales, la sobreingesta de alimento es frecuente en estas condiciones. De acuerdo con modelos animales, los endocanabinoides y sus receptores en el cerebro juegan un papel clave en la génesis y desarrollo de la obesidad. Se ha propuesto que los receptores a canabinoides CB1 (RCB1) expresados en el núcleo accumbens shell (NAcS) están involucrados en el incremento de las propiedades hedónicas del alimento. Para probar esta hipótesis, este estudio tuvo como objetivo evaluar los efectos de la activación de los RCB1 en el NAcS sobre la ingesta de alimento estándar durante la fase de luz del ciclo luz-oscuridad. Se evaluaron los efectos de la activación de los RCB1 con WIN 55-212-2 y CP 55,940 (0.125, 0.25, y 0.5 nmol) en el NAcS sobre la conducta alimentaria y la secuencia de saciedad conductual en ratas. Se encontró que ambos agonistas aumentaron la ingesta de alimento y demoraron la expresión de la saciedad durante la fase de luz. Lo anterior sugiere que los agonistas canabinoides estimulan el consumo de alimento cuando la motivación por el mismo es baja y la palatabilidad es normal.
Palabras clave: Canabinoides, alimentación, núcleo accumbens shell, secuencia de saciedad conductual.
Resumo
A obesidade e suas patologias relacionadas são riscos de saúde muito conhecidos. Ainda que a obesidade e o sobrepeso possuam causas multifatoriais, a sobre ingestão de alimento é frequente nestas condições. De acordo com modelos animais, os endocanabinóides e seus receptores no cérebro jogam um papel chave na gênese e desenvolvimento da obesidade. Foi proposto que os receptores a canabinóides CB1 (RCB1) expressos no núcleo accumbens shell (NAcS) estão envolvidos no aumento das propriedades hedônicas do alimento. Para testar esta hipótese, este estudo teve como objetivo avaliar os efeitos da ativação dos RCB1 nos NAcS sobre a ingestão de alimento padrão durante a fase de luz do ciclo luz-escuridão. Avaliaram-se os efeitos da ativação dos RCB1 com WIN 55-212-2 e CP 55,940 (0.125, 0.25, e 0.5 nmol) no NAcS sobre a conduta alimentar e a sequência de saciedade condutual em ratos. Encontrou-se que ambos agonistas aumentaram a ingestão de alimento e demoraram a expressão da saciedade durante a fase de luz. Isso sugere que os agonistas canabinóides estimulam o consumo de alimento quando a motivação pelo mesmo é baixa e a palatabilidade é normal.
Palavras chave: Canabinóides, alimentação, núcleo accumbens shell, sequência de saciedade conductual.
Obesity and its related pathologies are well-known health threats. Despite obesity and overweight have multifactorial causes, recurrent over-consumption of energy dense foods is frequently present in these conditions. Those diets are commonly highly palatable and may activate the brain reward circuit (Bassareo & Di Chiara, 1999; Mellis, et al., 2007), promoting over-consumption when these foods are freely available (Perello, Chuang, Scott & Lutter, 2010) or with limited access schedules (Berner, Avena & Hoebel, 2008; Dimitriou, Rice & Corwin, 2000).
The nucleus accumbens shell (NAcS) plays a key role in processing the rewarding properties of stimuli, and several neurochemical signaling pathways interact to regulate dopamine release in the NAcS and contribute to provide rewarding and/or hedonic properties to food (Gardner, 2005). The endocannabinoid system has been implicated in reward-driven feeding (Matias, Cristino & Di Marzo, 2008), and interestingly cannabinoid receptor 1 (CB1R) KO mice are resistant to diet-induced obesity, indicating that energy dense foods require endocannabinoid signaling to generate obesity (Quarta, et al., 2010). The experimental evidence indicates that the acute activation of CB1R in the NAcS by administration of anandamide (Soria-Gómez, et al., 2007) or 2-AG (Kirkham, Williams, Fezza & Di Marzo, 2002) consistently stimulates chow intake.
Previous results in our laboratory showed that activation of CB1R in the paraventricular nucleus of the hypothalamus prevented the natural satiety sequence by modulating homeostatic mechanisms (Escartín-Pérez, et al., 2009); nevertheless activation of cannabinoid receptors in the NAcS might also stimulate feeding by a different mechanism, for instance stimulating the hedonically positive sensory properties of food. Accordingly, the present study was aimed to evaluate the effects of activation of CB1R in the NAcS on feeding behavior using the behavioral satiety sequence (BSS) analysis. It is hypothesized that acute activation of CB1R in the NAcS will stimulate feeding when a standard chow food is offered during the light phase of the photoperiod, when the basal food intake is naturally low and the hedonically positive sensory properties of food may be stimulated by cannabinoid agonists.
METHOD
Subjects
A total of 38 male Wistar rats (UNAM, FESI México) weighting 220-240 g at the beginning sessions were used. Rats were housed in clear plastic cages with an inverted 12x12 h light and dark cycle with standard food (LabDiet® formulab diet #5008) and water ad libitum. All experimental procedures complied with the Official Mexican Norm (NOM-062-ZOO-1999), entitled Technical Specifications for the Production, Care, and Use of Laboratory Animals.
Instruments and materials
Drugs and treatments. CP 55 940 (non-selective CB1R agonist) and WIN 55 212-2 (non-selective CB1R agonist) (Tocris Cookson Inc., Ellisville, MO, USA). Both drugs were dissolved in DMSO, Tween-80 and 0.9 % saline solution (4:2:94 v/v/v). Different doses of drugs were administered intra-NAcS according to the experimental design in a volume of 0.5 μl at 0.2 μl/min. Fresh solutions of drugs were prepared before the injections.
Behavioral test (food intake and behavioral satiety sequence). Consumption of standard chow (g) was assessed in two independent periods of 60 min each (hour 1, hour 2), 10 and 70 min post injection. The analysis of the BSS was conducted in both 60 min observation periods, which were divided in 12 segments (5 min bins). The duration (s) of each behavior was measured from the videotape recording to the following mutually exclusive categories: Intake (standard food), resting (inactivity with or without closed eyes, head of the rat was on the cage floor), activity (any other behavior different to feeding, resting, and grooming including locomotion, smelling, sniffing, etc), and grooming (self-cleaning of any part of the body). Durations of each behavioral category are presented in terms of duration (±SEM) over 12 periods (5-min bins) per hour, and served as the measure of the progression of BSS (Escartín-Pérez, et al., 2009).
Histology. After the behavioral tests, rats were given a lethal dose of sodium pentobarbital and were beheaded, brains were removed and fixed in 10% formalin for two days. Brains were sectioned at 100 μm in the coronal plane, and then positions of the cannulas and injection sites were verified. Brains in which drugs were not administered in the NAcS were excluded from the present report (Figure 1).
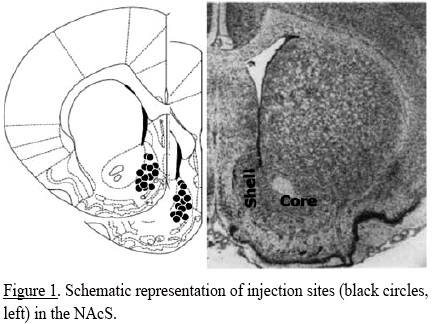
Procedure
Surgery. All animals were anaesthetized with ketamine and xilasine (112.5 mg/kg and 22.5 mg/kg I.P.) and were stereotaxically implanted (unilaterally) with guide cannulas for injection in the nucleus accumbens shell (coordinates AP +1.52 mm and ML -0.7 mm relative to bregma, DV -6.0 mm relative to dura mater) according to Paxinos and Watson's Atlas (1998). After surgery, animals were administered with benzathine penicillin (300,000 UI/kg, I.M.) and they had a surgical recovery period of five days with food and water ad libitum.
Design and recordings. After the surgical recovery period, animals were randomly assigned to independent groups that received vehicle or different intra-NAcS treatments of WIN 55 212-2 (0.125, 0.25, 0.5 nmol) or CP 55 940 (0.125, 0.25, 0.5 nmol) and behavioral recordings were conducted. Immediately after central injections, animals were placed into their home cages with pre-weighted standard chow; behavior was video-recorded and subsequently measured to obtain the BSS during two consecutive hours (two recordings of 60 min each, hour 1 and hour 2). At the end of the observations, food containers were removed and ingestion of food was calculated (g). Animals were individually housed with food and water ad libitum. All behavioral observations were conducted during the light phase of the light/dark cycle.
Statistical analysis. Analyses of data from food intake measurements were performed by two-way ANOVAs (dose x hour) to calculate significance of the differences by intra- NAcS injections. When appropriate, the Bonferroni's test was performed. The criterion for statistical significance was p < 0.05. Data were analyzed using GraphPad Prism Version 5.0.
RESULTS
Data from standard food consumption measurements (g) and the BSS (s) are expressed as means (± SEM) and are organized in two independent sections: 1) Food intake, and 2) Behavioral satiety sequence (BSS) analyses.
Effects of cannabinoid receptors activation in the NAcS on food intake
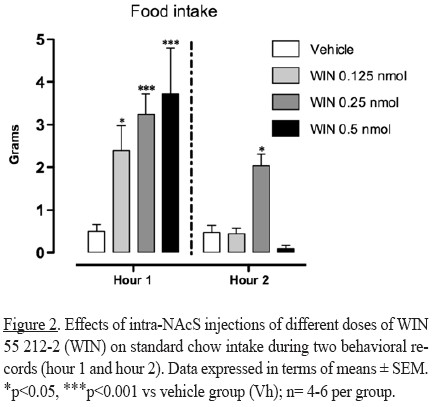
Firstly, it was investigated if intra-NAcS activation of cannabinoid receptors with two different agonists produced significant changes in chow consumption in a situation where the basal intake is low (light phase of photoperiod). Consistent with reports that found a stimulatory effect of activation of CB1R in the nucleus accumbens, intra-NAcS injections of WIN 55 212-2 (all doses) significantly increased standard chow intake (interaction dose x hour F(3,30)= 4.480; factor dose F(3,30)= 7.511; factor hour F(1,30)= 25.81;p< 0.05) during the first hour of observation, and the effect persisted during the second hour of the behavioral record when a dose of 0.25 nmol was administered (Figure 2).
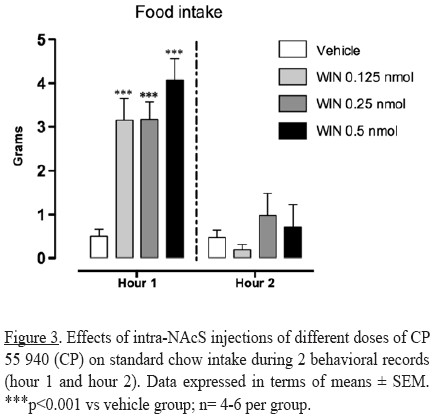
Independent groups of rats were administered with the non-selective cannabinoid receptor agonist, CP 55 940, at different doses. It was found that the activation of cannabinoid receptors in the NAcS (all doses of CP 55940) increased significantly standard chow intake (interaction dose x hour F(3,30)= 6.045 p<0.01; factor dose F(3,30)= 7.674 p<0.001; factor hour F(1,30)= 54.66;p< 0.001) during the first hour. No statistically significant effect on the second period of observation was found (Figure 3).
Effects of cannabinoid receptors activation in the NAcS on the behavioral satiety sequence
In order to assess the behavioral profile associated to the stimulatory effect of intra-NAcS cannabinoid receptor activation on chow intake, the BSS in the same periods of time (hour 1 and hour 2) was analyzed. It was found that control animals began the test eating during the first periods, but subsequently the time spent eating decreased while the time of resting increased, showing the transition from eating to resting after the third period of the first hour of observation; activity and grooming remained low during the complete test (hour 1 and hour 2). As expected, in the second hour, control animals spent time resting during almost the complete 60-min record, reflecting satiety (Fig. 4. A, B). Animals treated with 0.125 and 0.25 nmol of WIN 55 212-2 showed a delayed transition from eating to resting in the first hour of observation (6th and 7th periods, respectively), resting less time and with a higher level of activity (specially the 0.125 nmol dose) than control rats. During the second hour of observation, animals treated with the lower dose of WIN 55 212-2 (0.125 nmol) remained comparable to the control group; however, the 0.25 nmol dose induced new meals and a higher level of activity, preventing the increase of the resting time as observed in control animals (Figure 4. C, D, and E, F). Finally, the higher dose of WIN 55 212-2 did not alter the behavioral profile observed in the control group. Nevertheless, animals in this group spent more time eating during the beginning of the first hour (Figure 4. G, H).
The behavioral profile produced by intra-NAcS injections of CP 55 940 (0.125, 0.25 and 0.5 nmol) was also assessed and it was found that all doses consistently delayed the transition from eating to resting during the first hour of observation (after the 6th, 7th and 9th periods, respectively), with a corresponding increase on the time of activity (Figure 5. C, E, G). During the second hour of observation, the higher doses of CP 55 940 (0.25 and 0.5 nmol) decreased the time spent resting (Figure 5. F, H), while the lower dose (0.125 nmol) remained similar to the control group (Figure 5. D).
DISCUSSION
The present study was aimed to test the hypothesis that acute activation of CB1R in the NAcS stimulate standard chow intake during the light phase of the photoperiod in rats maintained on ad libitum chow. It is shown here that both WIN 55 212-2 and CP 55 940 increase standard chow intake and delay the expression of the natural satiety sequence. According to the behavioral analyses carried out, intra-NAcS administration of CB1R agonists stimulates feeding during the light phase of the photoperiod, when the basal intake is naturally low, suggesting that cannabinoid agonists consistently stimulate food consumption when motivation for food is low and palatability is regular (standard chow).
Different studies have explored the role of cannabinoid receptors in the hypothalamus (Verty, McGregor & Mallet, 2005; Jamshidy & Taylor, 2001), indicating that the stimulatory effect of cannabinoids is related to the homeostatic modulation of food intake. Furthermore, behavioral experiments have shown that rimonabant bilaterally microinjected into the NAcS selectively decreased highly palatable food intake in an operant task (Guegan, et al., 2013). Our results confirm and extend the previous evidence that intra-accumbens administration of anandamide or 2-AG increase standard chow consumption (Soria-Gómez, et al., 2007; Kirkham, et al., 2002) by a behavioral-specific mechanism. Taking together these findings, it seems possible that cannabinoid receptors modulate feeding behavior simultaneously by two independent processes; the first one, homeostatic, characterized by affecting energy balance and motivational processes (hunger, appetite, satiety) (Cota, et al., 2003; Escartín-Pérez, et al., 2009); and the second one, hedonic, increasing the rewarding properties of food and its hedonically positive sensory properties (Di Patrizio & Simansky, 2008).
Despite the fact that the lower doses of WIN 55 212-2 (0.125 and 0.5 nmol) and all doses of CP 55 940 increased the time of activity (especially in the first hour of observation), this effect did not disrupt the behavioral profile associated to post-ingestive satiety sequence either the transition from eating to resting, suggesting that the endocannabinoid system in this particular conditions promotes feeding and increases motor activity. It is well know that cannabinoids affect motor behavior via CB1R by regulating glutamatergic and GABAergic transmission (González, et al., 2009). Specifically, cannabinoid receptors located in basal ganglia modulate both the inhibitory and the excitatory neuronal transmission, providing regulation of movement (Sanudo-Pena, Patrick, Patrick & Walker, 1996). Several reports showed that systemic administration of the cannabinoid receptor agonist WIN 55,212-2 stimulated locomotor activity in the open field test at low doses (0.25-0.6 mg/kg) (Pandolfo, Pamplona, Prediger & Takahashi, 2007; Drews, Schneider & Koch, 2005) and this effect was described as a general motor stimulating effect. Nevertheless, in the present study both cannabinoid agonists were administered intra-NAcS and the behavioral profile elicited should not be considered as a general stimulating effect, since the endogenous cannabinoid system is strongly involved in the development of food-induced behavioral alterations, such as foodseeking. Supporting this notion, it was demonstrated that the deletion of CB1R decreased operant behavior and the motivation to obtain highly palatable food in CB1 KO mice (Guegan, et al., 2013). Furthermore, the findings that CB1R KO rodents are resistant to diet-induced obesity (Ravinet-Trillou, Delgorge, Menet, Arnone & Soubrié, 2004), and that over-activity of the endocannabinoid system promotes an obese phenotype (Maccarrone, et al., 2010), in conjunction with the cannabinoid-induced increase of activity observed in this study, suggest a functional connection between the endocannabinoid system, ineffective inhibition of responses and obesity. Accordingly, chronic exposure to cannabinoid agonists should be studied in order to determine if the cannabinoid-induced increase of activity is related to the impulsivity observed in obesity (Nederkoorn, Braet, Van Eijs, Tanghe & Jansen, 2006).
In spite of the pharmacological lack of selectivity of WIN 55-212-2 and CP 55,940 at CB1 and CB2 receptors, there is no knowledge of reports of CB2 expression in the NAcS. Only multifocal expression of CB2 immunoreactivity has been reported in different areas of rodent brains at levels much lower than those of CB1 receptors (cerebellum, hippocampus, olfactory tubercle, cerebral cortex, thalamus, amygdala, basal ganglia, periaqueductal gray) (Gong, et al., 2006). Nevertheless, conclusive pharmacological evidence of the CB1R participation in the reported effects should be provided (i.e. administering selective antagonists).
In summary, activation of CB1 receptors in the nucleus accumbens shell stimulates standard chow intake during the light phase of the photoperiod, when the basal food intake is naturally low and the hedonically positive sensory properties of food may be stimulated. Further research should explore in detail the relationship between the endocannabinoid system, impulsivity, and obesity.
REFERENCES
1. Bassareo, V. & Di Chiara, G. (1999). Modulation of feedinginduced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. European Journal of Neuroscience, 11(12), 4389-4397. [ Links ]
2. Berner, L. A., Avena, N. M. & Hoebel, B. G. (2008). Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity (Silver Spring) 16,1998-2002. [ Links ]
3. Cota, D., Marsicano, G., Tschöp, M., Grübler, Y., Flachskamm, C., Schubert, M., Auer, D., Yassouridis, A., Thöne-Reineke, C., Ortmann, S., Tomassoni, F., Cervino, C., Nisoli, E., Linthorst, A. C., Pasquali, R., Lutz, B., Stalla, G. K. & Pagotto, U. (2003). The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. Journal of Clinical Investigation, 112, 423-431. [ Links ]
4. Di Patrizio, N. V. & Simansky, K. J. (2008). Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. Journal of Neuroscience, 28(39),9702-9709. [ Links ]
5. Dimitriou, S. G., Rice, H. B. & Corwin, R. L. (2000). Effects of limited access to a fat option on food intake and body composition in female rats. International Journal of Eating Disorders, 28,436-445. [ Links ]
6. Drews, E., Schneider, M. & Koch, M. (2005). Effects of the cannabinoid receptor agonist win 55,212-2 on operant behavior and locomotor activity in rats. Pharmacology Biochemistry and Behavior, 80(1),145-150. [ Links ]
7. Escartín-Pérez, R. E., Cendejas-Trejo, N. M., Cruz-Martínez, A. M., González-Hernández B., Mancilla-Díaz, J. M. & Florán-Garduño, B. (2009). Role of cannabinoid CB1 receptors on macronutrient selection and satiety in rats. Physiology and Behavior, 96, 646-650. [ Links ]
8. Gardner, E. L. (2005). Endocannabinoid signaling system and brain reward: Emphasis on dopamine. Pharmacology, Biochemistry and Behavior, 81(2), 263-284. [ Links ]
9. Gong, J. P., Onaivi, E. S., Ishiguro, H., Liu, Q. R., Tagliaferro, P. A., Brusco, A. & Uhla, G. R. (2006). Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Research, 1071,10-23. [ Links ]
10. González, B., Paz, F., Florán, L., Aceves, J., Erlij, D. & Floran, B. (2009). Cannabinoid agonists stimulate [3H]-GABA release in the globus pallidus of the rat when Gi proteinreceptor coupling is restricted. Journal of Pharmacology and Experimental Therapeutics, 328, 822-828. [ Links ]
11. Guegan, T., Cutando, L., Ayuso, E., Santini, E., Fisone, G., Bosch, F., Martinez, A., Valjent, E., Maldonado, R. & Martina, M. (2013). Operant behavior to obtain palatable food modifies neuronal plasticity in the brain reward circuit. European Neuropsychopharmacology, 23(2), 146-159. [ Links ]
12. Jamshidy, N. & Taylor, D.A. (2001). Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. British Journal of Pharmacology, 134, 1151-1154. [ Links ]
13. Kirkham, T. C., Williams, C. M., Fezza, F., & Di Marzo, V. (2002). Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. British Journal of Pharmacology, 136(4), 550-557. [ Links ]
14. Maccarrone, M., Gasperi, V., Catani, M. V., Diep, T. A., Dainese, E., Hansen, H. S. & Avigliano, L. (2010). The endocannabinoid system and its relevance for nutrition. Annual Reviews of Nutrition, 30, 423-440. [ Links ]
15. Matias, I., Cristino, L. & Di Marzo, V. (2008). Endocannabinoids: Some like it fat (and sweet too). Journal of Neuroendocrinology, 20(1), 100-109. [ Links ]
16. Melis, T., Succu, S., Sanna, F., Boi, A., Argiolas, A. & Melis, M. R. (2007). The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neuroscience Letters, 419 (3), 231-235. [ Links ]
17. Nederkoorn, C., Braet, B., Van Eijs, Y., Tanghe, A. & Jansen, A. (2006). Why obese children cannot resist food: The role of impulsivity. Eating Behaviors, 7, 315-322. [ Links ]
18. Pandolfo, P., Pamplona, F. A., Prediger, R. D. & Takahashi, R. N. (2007). Increased sensitivity of adolescent spontaneously hypertensive rats, an animal model of attention deficit hyperactivity disorder, to the locomotor stimulation induced by the cannabinoid receptor agonist WIN 55,212-2. European Journal of Pharmacology, 563(1-3), 141-148. [ Links ]
19. Paxinos, G. & Watson, C. (1998). The brain in stereotaxic coordinates. New York: Academic Press. [ Links ]
20. Perello, M., Chuang, J., Scott, M. M. & Lutter, M. (2010). Translational Neuroscience approaches to hyperphagia. The Journal of Neuroscience, 30(35), 11549-11554. [ Links ]
21. Quarta C., Bellocchio L., Manzini G., Mazza R., Cervino C., Braulke L., Fekete C., Latorre R., Nanni C., Bucci M., Clemens L., Heldmaier G., Watanabe M., Leste-Lassere T., Maitre M., Tedesco L., FanelliF., Reuss S., KlausS., Srivastava R., Monory K., Valerio A., Grandis A., de Giorgio R., Pasquali R., Nisoli E., Cota D., Lutz B., Marsicano G. & Pagotto U. (2010). CB1 signaling in forebrain and sympathetic neurons is a key Determinant of endocannabinoid actions on energy balance. Cell Metabolism, 11, 273-285. [ Links ]
22. Ravinet-Trillou, C., Delgorge, C., Menet, C., Arnone, M. & Soubrié, P. (2004). CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. International Journal of Obesity, 28, 640-648. [ Links ]
23. Sanudo-Pena, M.C., Patrick, S. L., Patrick, R.L. & Walker, J.M. (1996). Effects of intranigral cannabinoids on rotational behavior in rats: Interactions with the dopaminergic system. Neuroscience Letters, 206, 21-24. [ Links ]
24. Soria-Gómez, E., Matías, I., Rueda-Orozco, P. E., Cisneros, M., Petrosino, S., Navarro, L. Di Marzo, V. & Próspero-García, O. (2007). Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. British Journal of Pharmacology, 151, 1109-1116. [ Links ]
25. Verty, A.N., McGregor, I.S. & Mallet, P.E. (2005). Paraventricular hypothalamic CB(1) cannabinoid receptors are involved in the feeding stimulatory effects of Delta(9)tetrahydrocannabinol. Neuropharmacology, 49 (8), 1101-1109. [ Links ]













