INTRODUCTION
Maternal Behavior (MB) is of vital importance in human being development and the development of other altricial species such as humans, rodents, some carnivorous and some omnivorous, as they are born underdeveloped to survive on their own. Thus, the earliest interpersonal relationship begin at birth, where mothers provide them the care necessary to survive and mature (Alsina-Llanes, Brun and Olazabal, 2015; Wickham et al., 2015). Thereby, emotional responses are also regulated, thus favoring adequate growth and cognitive development (attention, memory, learning, etc.) (Kristal, 2009).
Cocaine abuse in women of fertile age, 12 to 25 years, fluctuates between 10% and 15% during pregnancy (Eyler et al., 2009; Office of Applied Studies, 2007). This abuse has a high long term negative impact on mothers, children, families and society in general. Children of mothers addicted to cocaine, present numerous alterations to their emotion, cognition, motor skills, and behavior, such as anxiety, depression, stress response complications, emotional regulation difficulties, instability, irritability, attentional deficit, oppo sitional defiant disorder, lability, anorexia and drug abuse vulnerability (Ackerman, Riggins and Black, 2010; Bennett, Bendersky and Lewis, 2007; Coleman et al., 2014; Chaplin et al., 2014; Hancock and Grant, 2009; Kippin, Campbell, Ploense, Knight and Bagley, 2015; Lambert and Bauer, 2012; Lester et al., 2012; Lucantonio, Stalnaker, Shaham, Niv and Schoenbaum, 2012; Minnes, Singer, Meeyoung, Miaoping, Lang and Yoon, 2014; Richardson, Goldschmidt, Larkby and Day, 2013; Sinha, 2008).
Mothers addicted to cocaine present mother/child relationships characterized by low warmth, negligence, poor interaction (Williams and Johns, 2014), and insensibility to their children's basic needs: hunger, cleanliness, cold, protection and warmth due to the incapacity to understand their signals (Eiden, Schuetze and Coles, 2011). The interactions are unrewarding and cause more stress, additio nally, mothers receive poor or null postnatal care (Kippin, Campbell, Ploense, Knight and Bagley, 2015).
Cocaine is a substance that mainly blocks the recapture of Dopamine -DA-, Noradrenaline -NA- and Serotonin -5HT- (Colado and Alguacil, 2008; Tellez-Mosquera and Cote-Menendez, 2005; Williams, Lauder and Johns, 2011), and interacts with cholinergic systems -ACH- (Adinoff et al., 2010), opioids, glutamate, (Martinez-Raga, Knecht, Ramirez and Szerman, 2009), and y-aminobutyric acid -GABA- (Lester and Padbury, 2009; Olive, Koenig, Nannini and Hodge, 2001), in addition, it inhibits central and pe ripheral oxytocin production (Williams and Johns, 2014).
Oxytocin and dopamine systems are those that mainly mediate the filial, maternal, and social behavior, reward or reinforcement and reactivity to stress (Johns et al, 2005). Hence, cocaine abuse may be influencing the decrease of the maternal sensibility to their children's signals and thereby reducing maternal activity.
Additionally, the main neural circuits of parental behavior in humans are the limbic- hypothalamics: the amygdala, the insula, the hypothalamus, the ventral tegmental area, the striatum, and the medial prefrontal cortex. These circuits are altered by drug abuse (Johns et al, 2005) and result damaging mainly the prefrontal cortex, the orbital cortex and the anterior cingulate, involved in filial behavior and hyperreactivity to stress (Strathearn and Mayes, 2010). Thus, the baby is exposed from conception to the mother's physiological and behavioral imbalance, produced by the drug, and enhanced by her dysfunctional environment.
The effects of the drug on the developing baby, and on maternal behavior, as well as the diverse environmental factors that act on the dyad mother/child, complicate the identification of factors influencing the newborn neurodeve lopment as a consequence of cocaine abuse. Nevertheless, the animal models with rodents would allow to isolate the different variables and to know the impact hierarchy of the distinct factors involved, which facilitates an efective approach to this problematic.
Maternal Behavior (MB) in rodents begins at concep tion and continues to decline through to weaning (Alsina-Llanes, Brun and Olazabal, 2015; Carrera-Guermeur, 2007; Champagne and Meaney, 2007; Wang and Storm, 2011). Prior to birth it pertains to the preparation of the environ ment for the arrival of the ofspring, and the postpartum, triggered by endocrine and chemosensory signals such as smell, movement, and cry of the ofspring (Champagne et al., 2003; Wang and Storm, 2011), it presents a series of behaviors that guarantee the adaptation and survival of the offspring.
Various behavioral patterns of the mother have been identified in the postpartum, such as nest building, arching her back to give warmth and facilitate breastfeeding, licking, maternal grooming, and pup retrieval to return them to the nest, eating and drinking for the mother's self-maintenance and that of the offspring, self-grooming to eliminate possible noxious elements for the ofspring, locomotion or displacement, and rest (Alsina-Llanes, Brun and Olazabal, 2015; Angoa-Perez and Kuhn, 2015; Champagne et al., 2003; Kristal, 2009; Silverman, 1978; Wang and Storm, 2011).
Of all this broad range of behaviors, those that imply physical contact mother/offspring such as licking, breast feeding, heating, pup retrieval, amongst others, (proximal behaviors) have a strong impact on attachment or the re gulation of the rodents' bonding (Angoa-Perez and Kuhn, 2015; Champagne et al., 2003; Hertenstein et al., 2006; Pereira and Ferreira, 2015).
Variations in these behaviors, have repercussions for individual diferences that the ofspring show in their behavior and are linked to the responses of the hypothala-mic- pituitary- adrenal (HPA) axis, which is the main axis to respond to stress (Caldji, Diorio and Meaney, 2000). Mice that during infancy have poor licking from their mother are more fearful and beget more fearful ofspring and with more reactivity to stress (Champagne et al., 2003), while ofspring that receive more maternal licking have better memory and a higher hippocampus' development level. In this way, it can be seen that greater maternal contact reduces fear and anxiety reactions in ofspring (Hertenstein et al., 2006; Pereira and Ferreira, 2015).
MB is interdependent with the environment, environmen tal adversity (such as food shortage, predators' presence, etc.), increases the mother's anxiety, and decreases her response capacity; this in turn directly affects the development of the newborn's reactivity to stress (Caldji, Diorio and Meaney, 2000; Toth, 2015). Mothers in adverse circumstances take more time in pup retrieval; and pup licking and grooming decrease; these effects last to the 3rd generation (transge-nerational efects), even in the absence of any posterior stress (Champagne and Meaney, 2007; Champagne et al., 2003; Szyf, 2014; Toth, 2015).
Maternal deprivation alters the posterior HPA axis response to children's stress (Carrera-Guermeur, 2007; Frye et al., 2006). Primates and rodents subjected to high maternal deprivation are fearful, anxious, hold inappropriate social behavior, are high aggressive and have raised vulnerability to the abuse of substances such as ethanol (Sinha, 2008); morphine (Jaworski, Francis, Brommer, Morgan and Kuhar, 2005) and various drugs which are used to reduce anxiety (Kippin, Cambell, Ploense, Knight and Bagley, 2015; Sinha, 2008), additionally to numerous cognitive problems such as attention deficit, slow learning between others (Champagne et al., 2003; Stamatakis et al., 2015).
Cocaine injures MB, reduces maternal aggression: they are more submissive, attack less and threaten more. Even though the effects vary depending on the dose (Nephew and Febo, 2012; Williams and Johns, 2014), those under 30 mg/kg do not affect, or slightly delay the beginning but not maintenance if MB is already stablished (Nephew and Febo, 2012). 25 mg/kg, 13 mg/kg and 6.3 mg/kg cocaine increased rest away from the nest; the highest increase was observed in the higher dose and the smallest in the 6.3 mg/ kg dose, the others indexes such as nest building, latency to lie close to the ofspring and to pup retrieval were not affected (Nelson et al., 1998). However, McMurray (2011) found that 15 mg/kg/day of cocaine decreased behaviors aimed at ofspring and increased motor activity. Although mothers treated with cocaine (15mg/kg-1) returned the ofspring faster to the nest, when compared to those in the control group, without effects on the others indexes (Febo and Ferris, 2007).
High cocaine doses (higher or equal to 30 mg/kg) alter maternal behavior; decrease the sensibility to pups' signals for retrieval (Nephew and Febo, 2012). Cocaine 30 mg/kg/ day delayed the beginning of the MB, decreased frequency and duration of breastfeeding, warmth for offspring, licking and nest building, it also increased the latency of pup retrieval. The mothers subjected to this doses used more time in self-grooming, in environmental sniffing, in rearing and they stayed more time away from the nest (Johns et al., 2005).
Quiñones-Jenab, Batel, Schlussman, Ho and Kreek (1997) observed that the cocaine 45 mg/kg/day decreased the mothers' nest building ability, they did not choose suitable materials nor did they finish the construction of the nest. Doses cocaine 15mg/kg/twice a day (b.i.d.) of increased the latency to touch/sniff their pups, maybe because they reduced neonatal vocalizations (Lippard et al., 2015).
Williams and Johns (2014), in their review, found that cocaine altered the beginning of MB, depending on the doses and the administration regimen (chronic, acute or intermit tent), and its effect was seen in the first postpartum days.
The various results found with similar cocaine doses can be explain by the methodological variations such as drug administration routes, gestation time, as well as the species of mice used. Those results prevented knowledge of the impact of cocaine used during pregnancy to maternal behavior in mice.
The aim of this study was to assess the effects of chronic administration (from gestation day 8 until 21) of cocaine (25 mg/kg/day and 50 mg/kg/day) (s.c.) in postpartum maternal behavior in female CD1mice; analyzed with an frequency of occurrence of16 behavioral patterns observed from partum to weaning (20 consecutive days).
METHOD
A repeated-measures experimental design was used (3 x 20): two experimental groups with administration cocaine 25 mg/kg/day, and 50 mg/kg/day of, and one group with administration of saline solution, with twenty consecutive daily evaluations of MB.
Subjects
21 female CD1 pregnant mice, from the "Instituto Nacional de Salud" [National Health Institute], Bogota D.C. From gestation day six, they were housed individually, at the "Laboratorio de Psicología de la Universidad Católica de Colombia (LAPSUCC)" [Laboratory of Psychology of the Catholic University of Colombia], on a 12/12 light/ dark cycle, at a 23°± 2°C temperature, with free access to water and feed. 7 females were randomly assigned to each study group. The administration was subcutaneous (s.c.) for the control group and the groups that received cocaine (25 and 50nmg/kg/day). After birth, all the mothers stayed with their offspring until weaning, at 21 days.
Materials and instruments
Substances
25 mg/kg of cocaine, dissolved in 0.9% saline
50 mg/kg of cocaine, dissolved in 0.9% saline
0.9% saline solution
Substances were applied daily subcutaneously (s.c.) in a volume of 0.05 ml.
Cocaine was donated by the "Universidad Nacional de Colombia" [National University of Colombia] pharmaceutic analyses lab.
Ethogram ofpostpartum maternal behavior, adapted from Silverman (1978). This ethogram includes 16 behavioral patterns of postpartum maternal behavior, classified by proximal behaviors that implicate contact with the offspring, motor activity which includes animal movements to roam and recognize its space and self-maintenance associated to the mother's survival:
Proximal Behaviors. Breastfeeding: the mother lays on her side or on top of her offspring to breastfeed them. Nest repair: includes: nest building, in which the female presents a temporary and spatial organization of her territory, where she accumulates sawdust in a quadrant of the box forming the nest; nest moving, which consists in moving the location; cleaning nest, the mother eats the feces or throw them out of the nest with her back or front legs. Pup retrieval: the mother returns her pups to the nest holding them with her teeth by the neck, each time that any pup gets away from it. Maternal grooming: the mother cleans the pups' bodies with her tongue or legs. Warmth for offspring: the mother provides warmth to her pups by covering them with her body. Anogenital licking: the mother licks the abdominal, anal, genital, and urinary areas to stimulate the pup defecation and urination. Pups' body sniffing: the female tracks with her nose her pups' bodies.
Motor behaviors. Self-grooming: the female cleans her body with her tongue or her back or front legs. Environmental sniffing: the female sniffs the environment making head movements. Rearing: the female assumes a vertical position raising her front legs and holding herself on her back legs. Locomotion: the female moves across the cage floor. Climbing: the female walks clinging to the mesh tops of the cages with her legs.
Self-maintenance behaviors. Drink: the female laps from the spout. Eat: the female consumes food from the feeder. Rest: the female reposes with her eyes closed and remains still. Sleep: the female lies coiled up with her eyes closed.
Procedure
21 pregnant female CD1 mice, from the "Instituto Nacional de Salud", Bogota D.C. were used. They were kept individually from gestation day six, in the "LAPSUCC", on a 12/12 light/dark cycle, with controlled temperature condi tions of 23°± 2°C, and with free access to water and feed.
On the eighth gestation day they were weight in order to randomly assign seven females to each group to the subcutaneous (s.c.) saline, cocaine 25, 50 mg/kg/day admi nistration, which was performed through the double-blind method, that is to say, the correspondence of the dose that was administered was unknown. These substances were injected during the remaining 14 days ofgestation (G8-G21), in various places on the back of the neck to avoid necrotic skin lesions. After delivery, the females stayed with their offspring until weaning at day 21; during this time (20 first days) the interactions of each mother/offspring were video recorded for 15 minutes every day at the same time. The frequency of each behavioral index was recorded every 5 seconds, to get a full 15 minutes, on a modified ethogram based on the one created by Silverman (1978).
Ethical considerations
In respect to mice maintenance and management, the ethical regulations provided by Resolution N° 0088430 of October 4, 1993, issued by Ministry of Health of Colombia were consider. In accordance to said regulations, the animals were raised only for research, the minimum number of mice required to get scientifically approved results was used. The procedures of cocaine administration were performed by trained personnel and taking strict precaution to reduce pain and prevent mistreatment.
Data Analysis
The frequency of the diverse maternal behavior (MB) indexes postpartum were recorded every 5 seconds, for 15 consecutive minutes, during the 20 postpartum maternal behavior days. Initially, the MB indexes were examined with the repeated-measures Multivariate Analysis of Variance (MANOVA) 3 x 20, corresponding to three chronic cocaine administration groups CCA (0, 25, 50 mg/kg/day) and 20 consecutive observation days, with an alpha less than or equal to 0.05. A repeated-measures Analysis of Variance (ANOVA) was used on each index to review simple effects, to the post hoc the Tukey's Honestly-Significant-Difference (HSD) test was used. All the statistical analysis were done using SSPS version 20.
RESULTS
The results of the MB frequency comparisons will be presented, beginning with proximal behaviors, following with motor behaviors, and ending with self-maintenance behaviors.
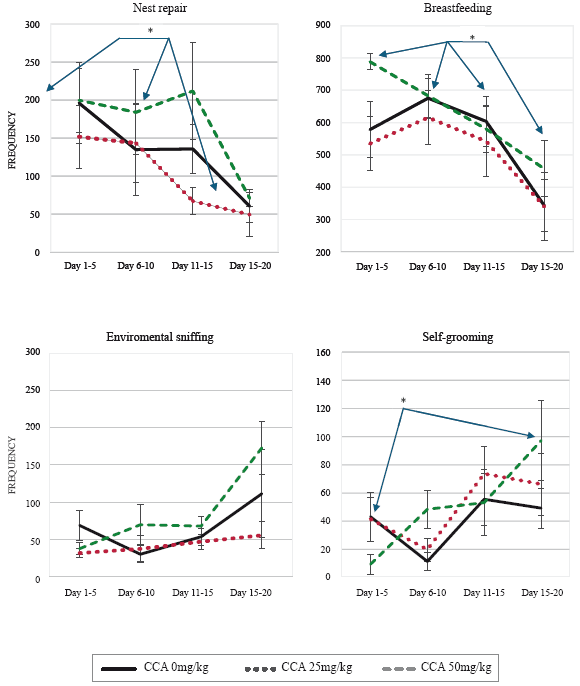
The preliminary analysis of proximal behavioral pat terns of MB frequency, such as anogenital licking, pups' body sniffing, and offspring carrying, had a low frequency of occurrence during the 20 days, so they were removed from the analysis.
Table 1 summarizes the MANOVA results, F values, degrees of freedom, and the p value of the 13 behavioral patterns, in the 20 observations performed of the MB in the four day groups 1-5, 6-10, 11-15 and 16-20. The main effects caused by chronic cocaine administration are represented with the initials CCA.
Table 1 MANOVA results for the thirteen behavioral patterns ofpostpartum maternal behavior in 20 consecutive observations and the 4 day groups.
According to MANOVA, no main effects were found in the frequencies of any of the MB indexes after the 20 daily observations were analyzed; significant differences were found only between the observations as through days [F (247,3591) = 1.83; p = 0.000], performed from postpartum to weaning.
When main effects were not found, the 20 observations of each behavioral pattern were grouped by 4, leaving 5 observations in each group, and they were analyzed using the repeated-measures MANOVA (see table 1).
Neither were main efects found, the diferences are still being determined amongst the observation days; in this case groups of [F (42,119) = 3.540; p = 0.000].
Repeated-measures ANOVA was used to analyze the variations of each MB index amongst the Chronic Cocaine Administration (CCA) groups, time and interaction; the Turkey HDS test was used for post hoc.
Significant decrease was found in nest repair [F (3,54) = 4.76; p = 0.012], through time in the three groups, according to the post hoc, it was in the last observation days (16-20), in comparison to the first 5 days (p=0.001) and to the 10 days (p=0.036), (Figure 1 and Table 2). Similarly, breastfeeding decreased significantly amongst the four groupings [F (3,54) = 7.90; p = 0.001], and the post hoc determined that it was in the last postpartum days (16-20), in comparison to the first 5 postpartum days (p = 0.021), to the 6 to 10 postpartum days (p = 0.019), and to the 11 to 15 days (p = 0.037) (see Figure 1).
Table 2 Repeated-measures ANOVA results to proximal behaviors: nest repair, breastfeeding, maternal grooming and warmth for offspring in 4 groupings.
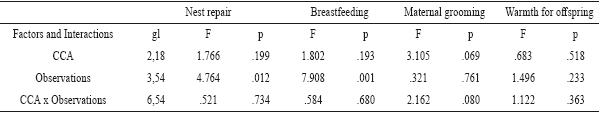
Note. Since the degrees of freedom (df) are similar to the indexes presented, they are described in the first column
Environmental sniffing increased significantly [F (3, 54) = 5.50; p = 0.015] between observations, although according to the post hoc, the required significance level was not reached (Figure 1), in the same way, self-grooming increased significantly in the last postpartum days [F (3,54)= 5.11; p= 0.003], in comparison to the first 4 postpartum days (p= 0.033), (see Figure 1, and Table 3).
Table 3 Repeated-measures ANOVA of motor behaviors: environmental sniffing, self-grooming, locomotion, rearing and climbing in 4 groupings.
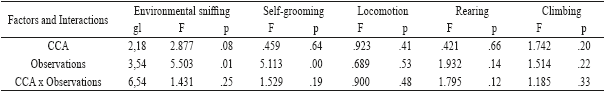
Note. Since the degrees of freedom (df) are similar to the presented indexes, they are described in the first column.
The others motor indexes did not vary significantly.
Self-maintenance indexes did not vary significantly (see table 4).
DISCUSSION
The purpose of the present study was to assess the results of chronic cocaine administration (in the last 14 gestation days) doses of 25 and 50 mg/kg/day s.c. on the postpartum maternal behavior frequency of occurrence observed during 20 consecutive days.
The chronic cocaine administration (applied since gestation day 8 until 21), neither the small dose 25 mg/kg/day nor the big dose 50 mg/kg/day affected the frequency of occurrence of either one of the 16 behavioral patterns that were determined in the postpartum MB. It did not affect the proximal behaviors frequency of occurrence, that are the ones directed to the offspring, or the motor activity classified as displacements or movements as either the self-maintenance ones, that are the ones directly involved with the mother's survival.
Aside from this high range of behavioral patterns, that in the present study integrates the postpartum MB, the MB had the possibility of occurrence, as was assessed, due to the fact that it was observed during 20 consecutive days until weaning that occurred on day 21. That is to say, MB was analyzed on its diferent postpartum phases, such as the beginning (postpartum days 1-5), the maintenance (postpartum days 6-14), and the decline (postpartum days 15-20), therefore, it can conclude that cocaine (25 and 50 mg/kg/day) during pregnancy efects were not found on the frequency of occurrence of postpartum MB.
The lack of CCA 25 mg/kg/day efects is consistent to obtained results in other researches, being that the small doses (less than 30 mg/kg) do not alter maternal behavior, or have light effects at the beginning, but not at the maintenance (Johns et al., 2005; Nelson et al, 1998; Nephew and Febo, 2012), or they did not afect any of the MB patterns; neither at the beginning, the maintenance, or the decline (Kinsley et al., 1994; Hess, Hahn, Benno and Schanz, 2002; Johns, Noonan, Li and Pedersen, 1994; Johns, Noonan, Zimmerman, Li and Pedersen, 1997; Kinsley, Turco, Bauer, Beverly and Wellman, 1994; Quinones-Jenab, Batel, Schlussman and Kreek, 1997).
Although in the McMurray (2011) and Caffrey and Febo (2014) studies, less than 15 mg/kg cocaine doses decreased important maternal behavioral parameters such as warmth for ofspring and responsivity to olfactory signals emitted by their offspring, in the present study warmth for offspring was not afected, and responsivity to the olfactory signals was not assessed.
As to the high doses (more than 30 mg/kg), it has been seen that cocaine disturbs MB. Most of the studies that assess cocaine in 30 mg/kg doses; found that it delays the beginning, duration, latency, and frequency of various parameters of maternal behavior (Johns et al., 2005).
In addition, in general terms, there is a lack of studies assessing cocaine use during gestation and the consequences to MB (Nephew and Febo, 2012); since the great majority are focused on the effects in the neurodevelopment of the ofspring of these mothers. The papers that have studied the efects of cocaine abuse with high doses, have more frequently used 30 mg/kg doses, since higher doses represent high toxicity for both mother and ofspring.
However, with higher cocaine doses than those used in the present study, of 60 mg/kg/day, no affection was found to the conditioning of place preference in the pups of mothers that had received this dose during gestation; instead, a lower cocaine dose -30 mg/kg/day- did (Dow-Edwards et al., 2014). If cocaine (60 mg/kg/day) did not have effects on the offspring, who are more vulnerable to be affected, since maternal behavior is more resistant to the effects of the drug, it could not affect maternal behavior, because numerous studies show that the doses that have been effective in pups, have not been effective in mothers (Nephew and Febo, 2012). This is because maternal behavior is too strong and resistant to numerous external factors, in this case to cocaine, due to the great importance that has on the survival of the species.
It is probable that cocaine in the both tested doses, does not alter the frequency of occurrence of these behavioral patterns, though it could disturb the molecular aspects of the behavior, such as latency, sequence, duration or the various characteristics of this pattern. Such would be the case of the milk provision when breastfeeding, the depth, shape and location of the nest inside the home-box, the retrieval velocity or the latency of pup retrieval, and the minimum distance needed for the mother to react retrieving them to the nest, or in the maternal licking difference between the pups and the pups body parts that she prefers to lick, such as head, body, genitals or abdomen (Champagne et al., 2003).
Another possibility would be that when cocaine admi nistration is suspended after birth, maternal behavior does not alter because the effects weaken in direct correlation to drug suspension (Johns et al., 2005), and the offspring, when lactating, stimulate the oxytocin production which in some way could reverse this hormone inhibition pro duct of cocaine (Johns, Noonan, Li and Pedersen, 1994). Nonewithstanding, seems like cocaine administration during the last 14 days of the 21 days of gestation, does not affect the frequency of occurrence of maternal behavior.
It is known that oxytocin and arginine vasopressin, relate positively to maternal behavior, in the same way that cocaine administered during pregnancy disturbs the arginine vasopressin signs and decrease oxytocin, addi tionally reducing maternal behavior (Nephew and Febo, 2012; Rodriguez-Borrero, Rivera-Escalera, Candelas, Montalvo, Muñoz-Miranda, Walker and Maldonado-Vlaar, 2010). Nevertheless, in the present study, cocaine did not affect the frequency of any MB index, therefore from the absence of visible effects in behavior, it can be inferred that cocaine (25 and 50 mg/kg/day) during approximately 70% of pregnancy did not affect neither oxytocin, nor va-sopressin because the groups exposed to cocaine and the control group behave in similar ways.
The present study focuses on the analysis of MB defined by a series of muscular patterns that shows a spatial-temporal process, and through them, access to the efects of cocaine; however, the fact of not having included neurochemical, electrophysiological correlations, does not allow a clear explanation for the lack of cocaine effects in the frequency of the diverse indexes of MB. As well as neither consents to explain why the modulate brain areas and the hormones that inflects in maternal behavior were not affected by the two cocaine doses tested. So it emphasizes in the results based on similar studies where you can compare the effects of determined doses, administration routes and cocaine administration periods during gestation.
It is worthwhile to highlight that the present study, in addition to having a wide range of behavioral patterns that formed the MB, only analyzed the frequency of occurrence every 5 seconds, which were finally totalized in 15 minutes. It would be convenient in future researches to include the latency, duration and sequence study of each one of these behavioral patterns, in order to notice small changes produce by cocaine during gestation.
As postpartum maternal behavior was analyzed in diferent phases, from beginning, to maintenance, to decli ne, which happens at weaning (Carrera-Guermeur, 2007; Champagne et al., 2003; Wang and Storm, 2011), it was found that proximal behaviors such as breastfeeding and nest repair, decreased significantly throughout the observa tions. And motor behaviors such as environmental sniffing and self-grooming, increased while locomotion, rearing and climbing did not change. Self-maintenance behaviors were stable throughout the observations, possibly due to their importance to both mother and ofspring survival, at least in the first days (Champagne et al., 2003), such as eat, drink, rest and sleep. As no cocaine effects were found, the variations that this study shows are normal in MB, in the phases of beginning, maintenance and decline.
Both the numerous indexes of MB assessed in the present study (16 behavioral patterns), as the observation period (20 consecutive days) were the two factors that prevented the comparison with other studies that had value the effects of cocaine administration in MB. Most of these researches are restricted to the analyses of determined indexes such as pup retrieval, breastfeeding, nest building to mention a few, and only during the first postpartum days (Nephew and Febo, 2012).
The lack of chronic cocaine administration effects in postpartum MB shows us the high resistance to the toxic effects of cocaine (25 mg/kg/day and 50 mg/kg/day) of this behavior, probably due to the great importance that these cares have on the survival of the species.
From the above, it can be concluded, that chronic cocaine administration (the last 14 gestation days) in doses of 25 mg/kg/day and 50 mg/kg/day, did not affect the frequency of occurrence of the different indexes of MB assessed during the time presented (the twenty days until weaning). Cocaine may possibly alter other characteristics of behavioral indexes, as occurrence of latency, duration or sequence of the diverse behavioral patterns.











 text in
text in