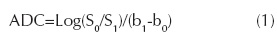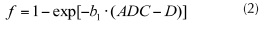Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Tecnura
Print version ISSN 0123-921X
Tecnura vol.18 no.spe Bogotá Dec. 2014
https://doi.org/10.14483/udistrital.jour.tecnura.2014.DSE1.a07
DOI: http://doi.org/10.14483/udistrital.jour.tecnura.2014.DSE1.a07
Potential application of ivim and dwi imaging in parkinson's disease
Aplicación potencial de imágenes ivim y dwi en la enfermedad de parkinson
Gloria M. Cruz*, Shengdong Nie**, Lijia Wang***
* MSc. Received her B.A degree in Biomedical Engineering at Universidad Antonio Nariño, Colombia, in 2000, the MSc. degree in Biomedical Engineer at The Beijing Institute of Technology, China, in 2010. She is currently a Senior Research Lecturer in Neuroscience and Neuro-computing and pursuing his PhD. degree within The University of Shanghai for Science and Technology. Her current researches interesting include Diffusion Tensor Imaging, Diffusion Weight Imaging, Perfusion Imaging, cancer and neuronal degenerative diseases. E-mail: g_milena@yahoo.com
** PhD. Received his Bachelor degree in Mathematics and Control Theory from Shandong University, China, in 1984. He was an assistant engineer in Ministry of Defense for Simulation System Laboratory and Department of Radiology at the Computer Medical College, during 1984-1990. He received his Master's degree in Automation Engineering from Electrical Shandong University of Science and Technology. He received his PhD degree in Biomedical Engineering from Jiaotong University, China, in 2000. He is now associate Professor and leader in University of Shanghai for Science and Technology at the Institute Medical Imaging Engineering. E-mail: nsd4647@163.com
*** PhD. She was born in 1984. She accepted her physics PhD degree from East China Normal University, China, on July, 2014. Her project is mainly focused on MRI techniques mainly including fully automated segmentation of cine cardiac MRI images. She also worked on fMRI study of visual illusory motion. Four paper and 7 patents have been published. Now she is working on MRI Pulse sequence design. E-mail: wanglijiamri@gmail.com
Fecha de recepción: June 10th, 2014 Fecha de aceptación: November 4th, 2014
Citation / Para citar este artículo: Cruz, G., Nie, S., & Wang, L. (2014). Potential application of IVIM and DWI imaging in Parkinson's disease. Revista Tecnura, 18 (Edición especial doctorado), 80-89. doi: 10.14483/udistrital.jour.tecnura.2014.DSE1.a07
Abstract
Parkinson's disease (PD) is a progressive degenerative neurological condition, which origin remains unclear. We are interested in proposing the study of blood flow in the substantia nigra (SN) in PD patients, based on findings that demonstrated relative hypoactivity in PD patients located to subthalamic nucleus and SN. It is believed that this hipoactivity may suggest changes in the blood flow to the SN, where the particular loss of dopaminergic neurons occurs.
The method used is the Incoherent Motion Intravoxel (IVIM) that allows measurement of blood flow to the microvascular level and recently has been producing high resolution quantitative perfusion maps.
This paper proposes to measure the perfusion in PD patients and find any correlation with neural activity and water displacements within the tissue. Assuming decreasing the local perfusion suggests the possible impairments that affect the neural activity in PD causing the progressive death of neurons in the SN.
Kewords: Diffusion Weighted Imaging (DWI), Intravoxel incoherent motion (IVIM),, Parkinson's disease (PD), Substantia nigra (SN).
Resumen
La enfermedad de Parkinson (EP) es una afección neurológica progresivamente degenerativa, con un origen aún desconocido. Estamos interesados en proponer el estudio del flujo sanguíneo en la sustancia negra (SN) en los pacientes con Parkinson, basados en hallazgos que han demostrado la relativa hipoactividad localizada en el núcleo subtalámico y la SN. Se cree que esta hipoactividad puede sugerir cambios en el fluido sanguíneo de la SN, donde ocurre la particular pérdida de neuronas dopaminérgicas.
El método a usar es el movimiento incoherente Intravoxel (IVIM) que permite la medición del flujo sanguíneo a nivel microvascular; además, recientemente ha producido una alta resolución de mapas de perfusión, permitiendo ser cuantificados.
El presente artículo plantea la posibilidad de medir la perfusión en pacientes con EP y encontrar la correlación de la actividad neuronal y de los desplazamientos de agua dentro del tejido a trevés de imágenes DWI e IVIM, suponiendo que la disminución de la perfusión local en la SN en los pacientes con EP interfiere en la actividad neuronal, siendo esta una de las posibles causas de la muerte progresiva de las neuronas en la sustancia negra.
Palabras clave: enfermedad de Parkinson (EP); Movimiento Incoherente Intravoxel (IVIM); Imágenes DWI; Sustancia negra (SN).
Introduction
Diffusion Weighted Imaging (DWI) (LeBihan et al., 1986) is a Magnetic resonance imaging (MRI) method, which allows the movements of the water molecules to be quantified (LeBihan, Moonen, van Zijl, Pekar, & DesPres, 1991), in vivo and non-invasively. Presently DWI has become an important technique in the characterization and detection of neurodegenerative diseases, such as PD (Köllensperger, Seppi, Liener, & Al, 2007; Meijer et al., 2013; Nicoletti et al., 2006; Seppi et al., 2006)
The movements of the water molecules, known as Brownian motion, is due to thermal agitation and is highly influenced by the cellular environment. Tirosh & Nevo (2013) suggest a possible reduction in water displacement that accompanies the neuronal activity, but neuronal activity was possibly affected by other physiological mechanisms as a consequence from Blood Oxygenation Level (BOLD) (Ogawa, Lee, Nayak, & Glynn, 1990) and blood flow. Otherwise, Kohno et al. (2009) suggest that the changes in DWI signal are not due to a BOLD effect because the diffusion response was less correlated with the changes in oxygenated, deoxygenated, and total hemoglobin. Even, they suggested that a decrease in water diffusion reflects early events that precede the vascular responses, which could originate from changes in the extravascular tissue (Kohno et al., 2009). Would be interesting to study on finding evidence to suggest that the decrease in the diffusion measure, really are influenced by the effects of perfusion in PD patients that result in neuronal loss and possible symptoms in PD. An example of evidence that suggest changes in the region of interest along a line between the SN and the lower part of the putamen and caudate complex of PD patients, in which most of the nigrostriatal dopaminergic neurons are included (Yoshikawa, 2004). Another important study by Vaillancourt et al., showed diffusion values in rostral, middle, and caudal region of the SN distinguishes in early stage PD patients from healthy controls (Vaillancourt et al., 2009), and structural abnormalities in the SN (Du et al., 2011; Péran, Cherubini, Assogna, & Al., 2010). Although the correlation between diffusion measures and pathological changes like perfusion in PD had not evidence, it is a primary concern for further researches to emphasize the importance of the SN.
BOLD functional MRI has great potential to serve as a non-invasive biomarker in PD (Prodoehl et al., 2010; Spraker et al., 2010) these studies showed hypoactives areas in response to a certain task in the basal ganglia, thalamus, cortical regions and SN. But BOLD is a method that faces several dependences with cerebral blood flow, cerebral blood volume, and blood oxygenation (Federau et al., 2012; Malonek et al., 1997). Also the spatial resolution is limited due to contribution from veins draining in the sides of activation (Turner, 2002). Generally we must use a series post-processing techniques to improve the spatial resolution. The recently advance techniques and functional imaging IVIM weighted MRI (LeBihan & Turner, 1992) could have a higher spatial correlation with perfusion and neural activation (Federau et al., 2012), even if we consider that IVIM concept has contributed to better understand the different vasculature components to the BOLD functional MRI signal (Le Bihan, 2012; Ogawa et al., 1993). Some of the others good examples or reasons that we encourage the use of IVIM is due to imaging demonstrated ischemic cerebral injury 2 hours post-occlusion in all cats, while T2-weighted imaging failed to show clear evidence of injury until 2-6 hours (Ide et al., 1993).
IVIM
IVIM weighted MRI (LeBihan & Turner 1992) measurements involve two movements in each voxel of biological tissue: the molecular diffusion and the microcirculation of water (known as perfusion). These two movements can be quantified in terms of the apparent diffusion coefficient (ADC) (LeBihan & Turner, 1992). IVIM images are generated from diffusion weighted images, with sequences that have different sensitivity to the type of vessels and also according to the sensitivity range of motion which is known as b values.
LeBihan (2008) indicated that low b values, less than 200 sec/mm², make the signal from large vessels with rapid disappearing flow rate; meanwhile higher b values above 200 sec/mm² make it possible to obtain signals of small vessels with slow flow that contributes to the acquisition of IVIM signal (LeBihan & Turner, 1992; LeBihan, 2008).
The apparent diffusion coefficient (ADC) can be calculated by using the equation (1):
Where b1 and b0 are the gradient factors of S1 and S0 sequences. According to this equation, IVIM theory can obtain the IVIM Image (which contains diffusion and perfusion) and the pure diffusion image called D.
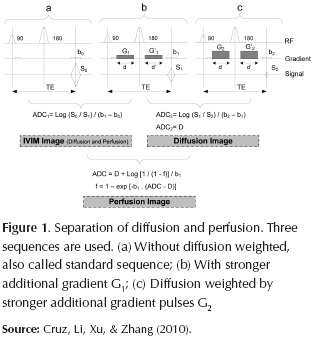
Based on Figure 1, an independent separation of D and f can be obtained from two IVIM images acquired at different gradient factors b. The separation and quantification of diffusion and perfusion images are calculated using third sequence S2 (LeBihan et al., 1988). This sequence, (c), is identical to the sequence S1, (b), except that the additional gradient pulse is stronger, and the echo attenuation due to diffusion is higher.
Combining the first IVIM image obtained from S0 (a) and S1 (b), and the pure diffusion image obtained from S1 (b) and S2 (c) is possible to obtain a pure perfusion image f as equation (2) follows:
Where b1 is the gradient factors of S1, ADC is the IVIM image obtained from S0 (a) and S1 (b), and D is the pure diffusion image obtained from S1 (b) and S2 (c).
PD
Initially described in 1817 by James Pakinson, PD is characterized by the selective loss of dopaminergic neurons in the SN zona compacta (SNc) (Braak, Rüb, Gai, & Del Tredici, 2003). The motor symptoms appear when at least 50-80% of dopaminergic neurons have died in the SN. This limits the effectiveness of potential treatments due to the low number of remaining neurons. According to Barcia et al. (2005) and Issidorides (1971), the EP can be related to disturbances of the microcirculation within the SN, which activates the pathophysiological cascade, which ultimately leads to the loss of dopaminergic neurons. However, another equally interesting theory by Kitagawa et al., related to changes in the cellular balance of antioxidants/oxidants due to increased free radicals in neurons (Kitagawa et al., 1990)They demonstrated that oxygen free radicals can be generated as a consequence of short periods of ischemia and neuronal death in vulnerable areas of the brain (Kitagawa et al., 1990; Sasaki et al., 2011)
In this work we propose to analyze IVIM technique to evaluate regional blood supply and diffusion measurements, and thus examine the correlation with regional atrophy in the SN of PD patients.
SN
SN has been divided into two macroscopic portions by their neurotransmitters, a dorsal portion rich in neurons containing neuromelanin called zona compacta (SNc) (Parent & Hazrati, 1995). The more ventral region is a portion with ovoid shape and lower neuron density called zona reticular (SNr), it has also a lateral portion. Functionally SN is divided in the inferior (caudal) and posterior (dorsal) in SNc; and the superior (rostral) and anterior (ventral) in SNr (Massey & Yousry, 2010).
Vascularization in SN
The SN is supplied with blood by four pial arteries: the basilar, the posterior cerebral, the posterior communicating and the choroid (Knox & Finley, 1936). Most of the parenchymal arteries supplying the SN also supply neighbouring nuclei (Cipolla, 2009; Knox & Finley, 1936). Within the SN, the capillary network of the SNc is much denser than in the SNr (Scheibel & Tomiyasu, 1980). SNc consists of rather densely arrayed vascular loops and whorls within which the neurons are situated. And SNr consists of longer blood vessels extending toward cerebral peduncle (Scheibel & Tomiyasu, 1980).
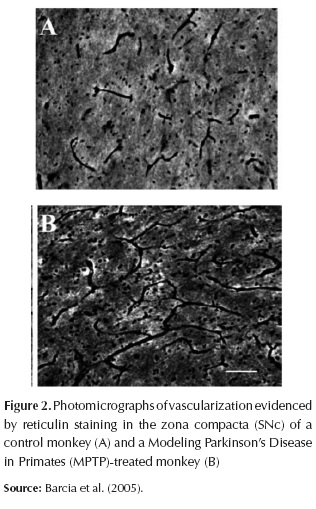
Observations were made of neurons of the SN by Issidorides (1971). He found that the melanin-containing neurons of the SNc in the normal brain have a close spatial relationship with the blood circulation. The capillary walls appear fused to the membranes of neuronal perikarya. In the Parkinsonian SNc since the close contact between nigral neuron and the capillary is lost (Issidorides, 1971). The width of this area ranged from a simple fissure, and seemed to increase with duration of disease. These findings suggest that this is due to the infiltration of the proliferated glia between cell surface and capillary wall (Issidorides, 1971; Hassler, 1938).
These changes in vascularization (Figure 2) may therefore modify the neuronal availability of blood nutrients, blood cells or toxic substances and neuronal susceptibility to parkinsonism (Barcia et al., 2005). Despite the increased number of blood vessels in the SN of the modeling PD in primates monkeys, they found no changes in the volume of the SNc (Barcia et al., 2005).
Within the SNc, the pathology is more pronounced in the caudal and the lateral portion (Hassler, 1938; Jellinger, 1986), in the vascularized portion by perforating arteries originating from the vascular territory of the perforating branches that originate from the basilar bifurcation and posterior cerebral artery, mesenphalic arteries. As same, others as dopaminergic midbrain nucleus German et al., 1989), in the locus ceruleus (Ohtsuka et al., 2013; Zweig et al., 1993), and monoaminergic neurons in the pons and medulla oblongata (Halliday et al., 1988).
PD has a high incidence in people over 50 years old with a slowly progressive course; consequently, multiple anatomical variations of the circle of Willis and/or structural changes in their perforating arteries, possibility can cause reduced blood flow to the SN, as well as others nucleus. Although the reduction in blood flow and nutrient delivery may initially be not clinically detectable, however, chronic persistence could ultimately to reduced thus compromising neuronal-glial interaction (De la Torre, 1994) Because the cerebral blood flow is normally regulated by the regional metabolic activity of neurons (Raichle et al., 1976). Recent research showed that cells implanted in the posterior region of the circle of Willis improved the severity of disease (Brazzini et al., 2010).
SN IN PD
In PD has the highest degree of neuronal loss in the SNc (Hirsch, Graybiel, & Agid, 1988), but it was more severely affected in the ventral (98%) than dorsal and lateral tiers (Fearnley & Lees, 1991). Another study with Neuromelanin-sensitive MRI was able to visualize changes associated with neuronal loss, the most significant signal attenuation was detected in the lateral part and most severely affected in PD (Ohtsuka et al., 2013).
The cause of the vulnerability of different regions is unknown. Apparently some of the reasons or features are because the neurons are more sensitive to oxidative stress in PD, which is known as the increased iron concentrations in the SN (EC Hirsch, 2009). Furthermore, an increased density of lactoferrin receptors has been reported on dopaminergic neurons and blood vessels (EC Hirsch & Faucheux, 1998; EC Hirsch, 2009).
Other a good correlation between PD patients and controls with the BOLD signal versus the performance of motor tasks and symptom-specific disease severity was made by Prodoehl et al. (2010) and Spraker et al., (2010). They showed the location on each anatomical region of the basal ganglia, primary motor cortex, supplementary motor area and SN were hypoactive in PD patients. Even the hypoactivity was observed in PD patients who had not yet started medication to subthalmic nucleus and SN (Spraker et al., 2010). In addition to these findings, one of the major responses that accompanies changes in neural activity is a localized change in cerebral hemodynamics: changes as cerebral blood flow, volume and blood oxygenation (Ogawa et al., 1993); also when a neuron increases the metabolic demands of oxygen and nutrients, they are extracted from the blood, meanwhile increases the local perfusion through vascular coupling (Roy & Sherrington, 1890).
Software application for ADC and IVIM
With the aim of implementing the IVIM theory above described and also trying to emphasize the rich information that ADC maps can give us, an "open source" application was implemented in order to generate ADC, diffusion and perfusion maps. The software was developed in Java language, as a plugin for ImageJ. ImageJ is a public domain Java image processing program (http://rsbweb.nih.gov/ij/). The logical structure of the program gives the user the possibility to perform two analyses: 1) IVIM Analysis: calculates the IVIM map, the pure diffusion map and the perfusion map; 2) ADC Analysis: calculates the ADC map for two or more slices by using a fitting algorithm.
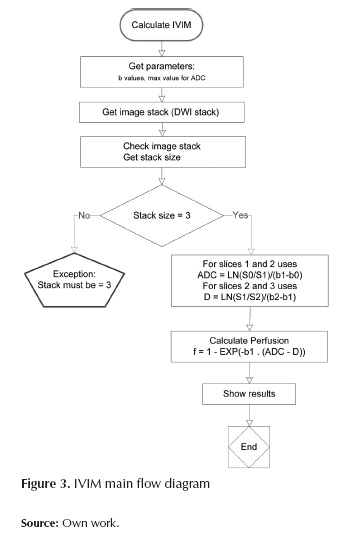
The following flow diagram (Figure 3) implements the main aspects of IVIM calculation:
On the other hand, the ADC analysis performs the ADC maps from multiple DWI slices with different b values. When two slices are introduced, with its corresponding b values, the software will perform an ADC analysis based on the equation (1). And, for more than two slices a fitting algorithm will be used. This fitting algorithm is based on the famous simplex method. The simplex algorithm, created by the American mathematician George Dantzig in 1947, is a popular algorithm for numerically solving linear programming problems. This method uses the concept of a simplex, which is a polytope of N + 1 vertices in N dimensions: a line segment in one dimension, a triangle in two dimensions, a tetrahedron in three-dimensional space, and so forth. Each ADC value in the map will be expressed in terms of 10-3 mm2/sec.
Conclusions
Basically the IVIM method an engineering point of view allows us to suggest that diffusion measures are excellent to proposing novel methods for development and planning of logic solutions to follow the disease progression and find new potential biomarkers in PD. Also authors such as Du et al. (2011) and Vaillancourt et al. (2009) proposed to improve and develop methods for separation of SN to distinguish SNc from SNr, as well as it is a necessary development novel tool for analyses and compare iron deposition, perfusion and diffusion within the SN, that can help to know the progression of the disease to understand the correlation between these biomarkers. The even other unclear boundary is between the sub-thalamic nucleus and the SN in the dorsal region (Du et al., 2011; Vaillancourt et al., 2009).
We can also use others different MRI techniques to obtain measures of perfusion information Calamante et al., 1999), that we don't propose in this paper, but they should be used with the same aim to understand the possible changes that produced the death of the dopaminergic neurons to avoid the appearance of the symptoms.
Diffusion Tensor Imaging (Mori & Zhang, 2006) provides excellent sensitivity and specificity in PD to identifier structural abnormalities in the SN (Du et al., 2011; Péran et al., 2010). These results were also consistent with the strong linear relationship with iron concentrations, particularly in the caudal region in SN, even between individual subjects (Vaillancourt et al., 2009). Another reason for this interesting research is the possible reduction in water displacement that accompanies the neuronal activity, which was possibly affected by the blood oxygenation level and blood flow (Tirosh & Nevo, 2013). In summary, our objective is to identify markers that can aid in diagnosis, disease progression monitoring and long-term drug impact analysis.
The effectiveness of the software depends on the correct acquisition of images. Due to the difficulty of getting good quality diffusion images in the brain with PD, the actual results lack of statistical support about the effectiveness of IVIM method. For that reason, this work only can present the software for further studies, which is expected the possibility to have more image data. The images used to test the IVIM software are just simple testing images. We want to open the possibility to use this application for future research.
Actually the software deficiency of preprocessing to reduce the noise, deblurring and adjust contrast, this is because the aim of this software was to develop an algorithm to perform IVIM theory; the focus was to implement the mathematical processing. In future stages preprocessing tools can be developed. And others imaging computational methods and software tools can improve the analysis in future research that are widely used to process and analyze DWI data (Hasan, Walimuni, Abid, & Hahn, 2011).
ACKNOWLEDGMENTS
The authors thank Chinese Scholarship Council award.
References
Barcia, C. et al. (2005). Changes in vascularization in substantia nigra pars compacta of monkeys rendered parkinsonian. Journal of Neural Transmission, 112, 1237-1248. doi:10.1007/s00702-004-0256-2. [ Links ]
Braak, H., Rüb, U., Gai, W., & Del Tredici, K. (2003). Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transmission, 110(5), 517-36. [ Links ]
Brazzini, A. et al. (2010). Intraarterial autologous implantation of adult stem cells for patients with Parkinson disease. Journal of Vascular and Interventional Radiology: JVIR, 21(4), 443-51. doi:10.1016/j.jvir.2010.01.008. [ Links ]
Calamante, F. et al. (1999). Measuring cerebral blood flow using magnetic resonance imaging techniques. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 19, 701-735. doi:10.1097/00004647-199907000-00001. [ Links ]
Cipolla, M. (2009). The Cerebral Circulation. In S. R. (CA): M. & C. L. Sciences (Ed.), The Cerebral Circulation. [ Links ]
Cruz, G., Li, Q., Xu, L., & Zhang, W. (2010). Differentiation of diffusion coefficients to distinguish malignant and benign tumor. Journal of X-Ray Science and Technology, 18(3), 235-49. doi:10.3233/XST-2010-0257. [ Links ]
De la Torre, J. C. (1994). Impaired brain microcirculation may trigger Alzheimer's disease. Neuroscience and Biobehavioral Reviews. doi:10.1016/0 149-7634(94)90052-3. [ Links ]
Du, G. et al. (2011). Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson's disease. Movement Disorders, 26(9), 1627-1632. [ Links ]
Fearnley, J., & Lees, A. (1991). Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain, 114, 2283-2301. [ Links ]
Federau, C. et al. (2012). Quantitative Measurement of Brain Perfusion with Intravoxel, 265(3). [ Links ]
German, D. et al. (1989). Midbrain dopaminergic cell loss in Parkinson's disease: computer visualization. Ann Neurol, 26(4), 507-14. [ Links ]
Halliday, G. et al. (1988). Distribution of monoamine-synthesizing neurons in the human medulla oblongata. J Comp Neurol, 273(3), 301-17. [ Links ]
Hasan, K. M., Walimuni, I. S., Abid, H., & Hahn, K. (2011). A review of diffusion tensor magnetic resonance imaging computational methods and software tools. Computers in Biology and Medicine, 41(12), 1062-1072. doi:10.1016/j.compbiomed.2010.10.008. [ Links ]
Hassler, R. (1938). [Zur Pathologie der Paralysis agitans und des Postenzephalitischen Parkinsonismus]. J Psychol Neurol, 48, 387-476 [in German] [ Links ].
Hirsch, E. (2009). Iron transport in Parkinson's disease. Parkinsonism & Related Disorders, 15 Suppl 3, S209-S211. doi:10.1016/S1353-8020(09)70816-8. [ Links ]
Hirsch, E. & Faucheux, B. (1998). Iron metabolism and Parkinson's disease. Mov Disord, 13(1), 39-45. [ Links ]
Hirsch, E. Graybiel, A. & Agid, Y. (1988). Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature, 334(6180), 345-348. [ Links ]
Ide, H. et al. (1993). Correlation between somatosensory-evoked potentials and magnetic resonance imaging of focal cerebral ischemia in cats. Surgical Neurology, 40, 216-223. doi:10.1016/0090-3019(93)90070-H. [ Links ]
Issidorides, M. R. (1971). Neuronal vascular relationships in the zona compacta of normal and parkinsonian substantia nigra. Brain Research, 25(2), 289-299. doi:10.1016/0006-8993(71)90439-2. [ Links ]
Jellinger, K. (1986). Overview of morphological changes in Parkinson's disease. Adv. Neurol, 45, 1-18. [ Links ]
Kitagawa, K. et al. (1990). Free radical generation during brief period of cerebral ischemia may trigger delayed neuronal death. Neuroscience, 35(3), 551-558. doi:10.1016/0306-4522(90)90328-2. [ Links ]
Knox, H. & Finley, M. D. (1936). Angio-architecture of the substantia nigra and its pathogenic significance. Arch NeurPsych, 36(1), 118-127. [ Links ]
Kohno, S. et al. (2009). Water-diffusion slowdown in the human visual cortex upon visual stimulation precedes vascular responses. Neuroscience Research, 65, S131. doi:10.1016/j.neures.2009.09.634. [ Links ]
Köllensperger, M., Seppi, K., Liener, C. & Al, E. (2007). Diffusion weighted imaging best discriminates PD from MSA-P: A comparison with tilt table testing and heart MIBG scintigraphy. Mov Disord, 22(12), 1771-1776. [ Links ]
Le Bihan, D. (2012). Diffusion, confusion and functional MRI. NeuroImage, 62(2), 1131-1136. doi:10.1016/j.neuroimage.2011.09.058. [ Links ]
LeBihan, D. & Turner, R. (1992). The capillary network: a link between IVIM and classical perfusion. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 27(1), 171-178. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1435202. [ Links ]
LeBihan, D. (2008). Intravoxel Incoherent Motion Perfusion MR Imaging: A Wake-Up Call. Radiology, 249(3), 748-752. [ Links ]
LeBihan, D. et al. (1988). Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology, 168, 497-505. doi:10.1148/radiology.168.2.3393671. [ Links ]
LeBihan, D. et al. (1986). MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology, 161(2), 401-407. [ Links ]
LeBihan, D. et al. (1991). Measuring random microscopic motion of water in tissues with MR imaging: A cat brain study. J Comput Assist Tomogr, 15(1), 19-25. [ Links ]
LeBihan, D., & Turner, R. (1992). The capillary network: A link between IVIM and classical perfusion. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 27(1), 171-178. [ Links ]
Malonek, D. et al. (1997). Vascular imprints of neuronal activity: Relationships between the dynamics of cortical blood flow, oxygenation, and volume changes. Proceedings of the National Academy of Sciences, 94 14826-14831. [ Links ]
Massey, L. & Yousry, T. (2010). Anatomy of the substantia nigra and subthalamic nucleus on MR imaging. Neuroimaging Clinics of North America, 20(1), 7-27. doi:10.1016/j.nic.2009.10.001. [ Links ]
Meijer, F. J. et al. (2013). Update on diffusion MRI in Parkinson's disease and atypical parkinsonism. Journal of the Neurological Sciences, 332(1-2), 21-9. doi:10.1016/j.jns.2013.06.032. [ Links ]
Mori, S. & Zhang, J. (2006). Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron,51, 527-539. [ Links ]
Nicoletti, G. et al. (2006). Imaging of Middle Cerebellar Peduncle Width: Differentiation of Multiple System Atrophy from Purpose: Methods: Results: Conclusion: Radiology, 239(3), 825-830. [ Links ]
Ogawa, S., Lee, T. M., Nayak, A. S. & Glynn, P. (1990). Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magnetic Resonance in Medicine, 14, 68-78. doi:10.1002/mrm.1910140108. [ Links ]
Ogawa, S. et al. (1993). Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophysical Journal, 64, 803-812. doi:10.1016/S0006-3495(93)81441-3. [ Links ]
Ohtsuka, C. et al. (2013). Changes in substantia nigra and locus coeruleus in patients with early-stage Parkinson's disease using neuromelanin-sensitive MR imaging. Neuroscience Letters, 541, 93-98. doi:10.1016/j.neulet.2013.02.012. [ Links ]
Parent, A. & Hazrati, L.-N. (1995). Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidium in basal ganglia circuitry. Brain Research Reviews, 20(1), 128-154. doi:10.1016/0165-0173(94)00008-D. [ Links ]
Péran, P., Cherubini, A., Assogna, F. & Al., E. (2010). Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain, 133, 3423-3433. [ Links ]
Prodoehl, J. et al. (2010). Blood oxygenation level-dependent activation in basal ganglia nuclei relates to specific symptoms in de novo Parkinson's disease. Mov. Disord, 25(13), 2035-2043. doi:10.1002/mds.23360.Blood. [ Links ]
Raiclhe, M. et al. (1976). Correlation between regional cerebral blood flow and oxidative metabolism. In vivo studies in man. Arch Neurol, 33(8), 523-526. [ Links ]
Roy, C. S. & Sherrington, C. S. (1890). On the Regulation of the Blood-supply of the Brain. The Journal of Physiology, 11, 85-158.17. doi:10.1152/japplphysiol.00257.2010. [ Links ]
Sasaki, T. et al. (2011). SIK2 Is a Key Regulator for Neuronal Survival after Ischemia via TORC1-CREB. Neuron, 69, 106-119. doi:10.1016/j.neuron.2010.12.004. [ Links ]
Scheibel, A. B. & Tomiyasu, U. (1980). A dendritic-vascular relationship in the substantia nigra. Experimental Neurology, 70(3), 717-720. doi:10.1016/001 4-4886(80)90198-3. [ Links ]
Seppi, K. et al. (2006). Progression of putaminal degeneration in multiple system atrophy: A serial diffusion MR study. NeuroImage, 31(1), 240-245. doi:10.1016/j.neuroimage.2005.12.006. [ Links ]
Spraker, M. B. et al. (2010). Basal ganglia hypoactivity during grip force in drug naïve Parkinson's disease. Human Brain Mapping, 31(12), 1928-1941. doi:10.1002/hbm.20987. [ Links ]
Tirosh, N. & Nevo, U. (2013). Neuronal activity significantly reduces water displacement: DWI of a vital rat spinal cord with no hemodynamic effect. NeuroImage, 76, 98-107. doi:10.1016/j.neuroimage.2013.02.065. [ Links ]
Turner, R. (2002). How Much Cortex Can a Vein Drain? Downstream Dilution of Activation-Related Cerebral Blood Oxygenation Changes. NeuroImage, 16(4), 1062-1067. doi:10.1006/nimg.2002.1082. [ Links ]
Vaillancourt, D. et al. (2009). High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology, 72(16), 1378-1384. [ Links ]
Yoshikawa, K. (2004). Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. Journal of Neurology, Neurosurgery & Psychiatry, 75(3), 481-484. doi:10.1136/jnnp.2003.021873. [ Links ]
Zweig, R. et al. (1993). The locus ceruleus and dementia in Parkinson's disease. Neurology, 43(5), 986-991. [ Links ]