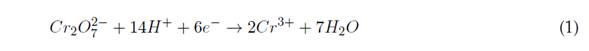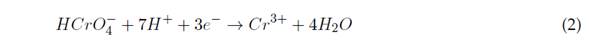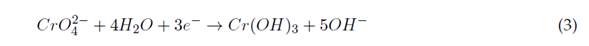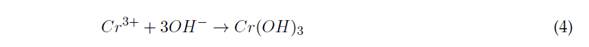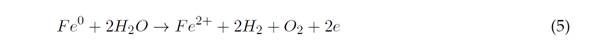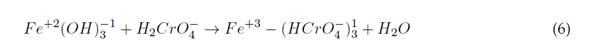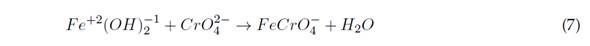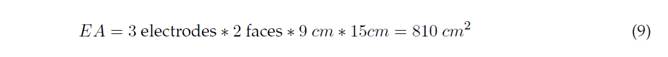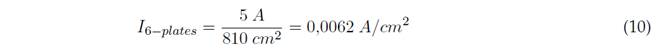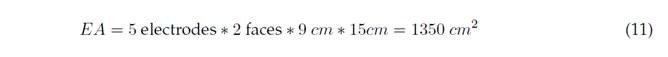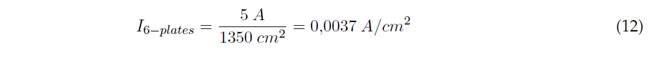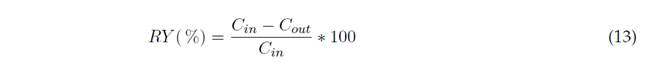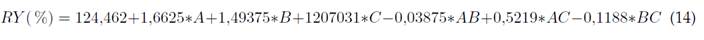INTRODUCTION
Chromium is a metal highly used in industrial processes such as printed circuit board manufacturing, tanning, metal processing, electroplating, and metal finishing. It usually exists in trivalent and hexavalent forms in aqueous solutions (Thirugnanasambandham & Shine, 2018). Hexavalent chromium (Cr(VI)), a carcinogen, is toxic to all forms of life and highly soluble in water (Mahmad et al., 2016). On the other hand, trivalent chromium (Cr(III)) has a low solubility in aqueous media, and it easily precipitates at pH >4 as Cr(OH)3 (Genawi et al., 2020).
In the case of wastewaters, Cr(VI) can be removed with several techniques, such as ion exchange, chemical reduction followed by precipitation, reverse osmosis, photocatalytic processes, and adsorption (Nwabanne et al., 2018). The mainstream treatment application currently used to eliminate Cr(VI) is its reduction to Cr(III) (Aoudj et al., 2017).
Electro coagulation (EC) is based on the creation of the coagulant while the sacrificial anode is degraded due to the applied current. At the same time, hydrogen is formed at the cathode, thus enabling the removal of contaminants by precipitation and flotation (Elabbas et al., 2016). This technology combines three interdependent processes, which operate jointly to eliminate contaminants: electrochemistry, coagulation, and hydrodynamics. Equations (1) to (4) show the reactions that occur during the removal of Cr(VI) with an Al(III) electrode (Sadeghi et al., 2017).
After the reduction reactions presented above, precipitation of Cr3+ in the form of hydroxide occurs:
It can be added that Al(OH)3, if generated from the reaction between Al3+ and OH− ions, is produced on the surface of electrodes. Secondary reactions can occur at the anode if it is powerful enough, such as the reduction of organic compounds by oxidation and Cl− present in the effluents. The Al(OH)3 flocs act as adsorbents or traps for the metal ions and thus remove them from the aqueous media. Additionally, a direct electrochemical reduction of Cr(VI) to Cr(III) can occur on the cathode’s surface (Singh et al., 2018). At the same time, hydroxyl groups formed at the cathode increase the electrolyte’s pH and can induce co-precipitation of Cr(III) as hydroxides (Ziati et al., 2018). This happens coordinately to eliminate contaminants present in the water.
Initially, when using stainless steel as an electrode, there is a process of electrogenerated species of Fe(II), as described in Equation (5):
This leads to Cr(VI) reduction to Cr(III) at pH between 2 and 6,5, and the precipitation of Cr(III), produced at pH >7,0 (Elabbas et al., 2020). This process can occur with Al(III) electrodes, and its reaction mechanism is summarized in Equations (1) and (2) (Ali Maitlo et al., 2019). The aforementioned reduction is evidenced by the fact that it takes an acidic medium and a source of Al(III) to break the balance to the right side (Elabbas et al., 2020). The interaction of HCrO4 - and CrO4 2- ions with iron oxides results in the formation of mono- and bidentate complexes on the surface of the internal sphere. This mechanism is described in Equations (6) and (7)(Ali Maitlo et al., 2019):
EC is a technique with an advantage over conventional coagulation due to lower investment costs, maintenance, energy expenditure, efficiency at low concentrations, lower sludge generation, and improved mud quality (Peng & Guo, 2020). Therefore, it has been widely implemented in the removal of Cr(VI) using electrodes of different nature. Prasetyaningrum et al. (2018) reported a 26% Cr(VI) removal efficiency using Al(II) electrodes for 2 h. Heffron et al. (2016) found an efficiency of 85% using Fe(II) electrodes. Similarly, Ali Maitlo et al. (2019) achieved a 100% removal rate after 4 h of operation. Thus, the objective of this study was to evaluate chromium elimination from synthetic solutions through the electrocoagulation method, using aluminum and stainless steel electrodes simultaneously. The effect of voltage variation, number of electrodes, and residence time was determined in this study.
METHODOLOGY
Potassium chromate (K2CrO4) (PanReac) was used at 98% purity as a reagent in the preparation of the synthetic solution, as well as 1,5-Diphenylcarbazide as a color indicator in the detection of Cr(VI) in solutions. The following equipment was also used: an HM Digital Conductivity Meter Aqua Pro 2 (0-9999 μS 0-80°C ± 2%); a Hanna Instruments portable pH-meter, model HI 9126 with a range of 2-16 pH ± 0,01 pH; a regulated PHYWE DC-Constanter power supply (0-30 V, 0-20 A); and a Meihua Biobase UV-Vis spectrophotometer, model UV-BK1900 with a 1 cm quartz cell.
Experimental design
A factorial design of experiments was used. The effect of voltage (20 and 30 V), the number of electrodes (6 and 10 electrodes), and the time of residence (20 and 30 min) were considered as independent variables. The tests were carried out in duplicate, for a total of 16 experiments.
Preparation of the solution
The synthetic solution of Cr(VI) at 50 mg/L was prepared by dissolving 0,1414 mg of K2CrO4 per liter of water (Tejada-Tovar et al., 2020).
Electrochemical cell design
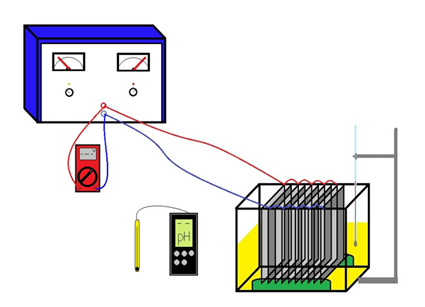
The design of the cell was made according to Al-Qodah and Al-Shannag (2017). Considering these criteria, a glass cell was built with a thickness of 4 mm and dimensions of 16 cm high, 16 cm wide, and 20 cm long, with a 3 L treatment volume of the cell. This design has the possibility of coupling 10 equidistant electrodes at 1,5 cm. The shape of the electrodes was a square plate of 15 x 15 cm. There were 5 stainless steel electrodes (AISI 316L, caliber 20, 0,91 mm thickness), which acted as inert material in the cathode; and 5 aluminum electrodes (caliber 16, 1,67 mm of thickness) as a sacrificial anode. The electrical connection to the PHYWE DC-Constanter power supply was made in a parallel monopolar configuration. The diagram of the electrocoagulation cell is shown in Figure 1.
The current density (I) was determined according to Equation (8):
The effective area (EA) of the anode is the sum of the exposed (immersed) areas of each electrode of the electrocoagulation cell. Out of each 15 cm of high plate, 9 cm are in contact with the water to be treated. Thus, for 6 electrodes, we have an effective area and applied current, as shown in Equations (9) and (10):
For 10 electrodes, we have the effective area and applied current, as shown in Equations (11) and (12):
Electrocoagulation experiments
Once the equipment was prepared, 3 L of Cr(VI) solution at 50 ppm were added, evaluating the effect of the variables according to the proposed experimental design. After the treatment time had elapsed, the plates were removed from the reactor and left to decant for 30 minutes. The final concentration of Cr(VI) in the solution was determined by UV-Vis spectrophotometry at 540 nm, following the standard method for the determination of hexavalent chromium in water, by means of the colorimetric complex formed between 1,5-Diphenylcarbazide and the ion (ASTM, 2017). The percentage of removal (RY) was obtained with Equation (13):
Where CCr(in) is the initial chromium concentration, and CCr(fin) is the post-treatment contaminant concentration in mg/L.
RESULTS
According to Figure 2, the experimental treatments had a variation in the percentage of removal from 60,15 to 92,9%, with the lowest performance using 10 electrodes, 30 V, and 20 min, while the best experimental condition was at 10 electrodes, 30 min, and 20 V. This removal is due to the fact that the hydrolysis products of aluminum and iron in steel destabilize the Cr(VI) in the solution, which allows agglomeration and a greater separation of the solution by sedimentation or flotation (Prasetyaningrum et al., 2018).
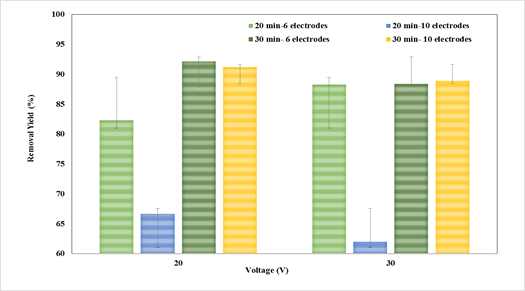
Source: Authors
Figure 2 Effect of the number of electrodes, contact time, and voltage variation on the efficiency of Cr(VI) removal
It has been reported that chromium removal using aluminum electrodes reaches approximately 72,65%, whereas, with stainless steel electrodes, a maximum removal efficiency of 88,35% is obtained, with the EC process being dependent on the pH, thus obtaining the best performance at acidic pH (3) (Mahmad et al., 2016). Aluminum, steel, and a combination of aluminum-steel electrodes have also been used, finding the best performance with aluminum electrodes. However, they reached only 26% performance during 2 h of operation, which is far below the results obtained in this research (Prasetyaningrum et al., 2018). The good performance in the removal process of Cr(VI) obtained in this research could be due to the connection between the electrodes (aluminum to the anode and steel to the cathode) because connecting both metals to the cell increases the presence of iron ions, aluminum, and its hydroxides produced by the hydrolyzation of the metallic plates (Khan et al., 2019). Previous studies have shown that the efficiency of Cr(VI) removal using electrochemical methods is 100 times better than using precipitation (He et al., 2020). It has been reported, by varying the voltage in 3, 6, and 9 V, that the removal efficiency increased to 34,95, 67,357, and 67,99%, respectively, thus demonstrating the influence of this variable on the efficiency of the process (Pavithra et al., 2020).
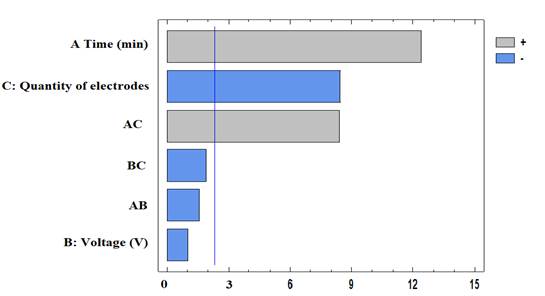
The standardized Pareto chart is shown in Figure 3, where it is evident that the residence time has a positive and significantly influential effect on the efficiency of Cr(VI) removal through EC.
From Figure 3, it can be stated that the increase in the number of electrodes has a negative effect, which is reflected in a lower efficiency when using 10 electrodes, 30 V, and 20 min. On the other hand, voltage variation does not significantly influence the process. It was observed that the simultaneous increase in contact time and the number of electrodes benefits removal efficiency. This could be due to the presence of Fe(II) and Al(III) hydroxides, which increases with the number of electrodes. These compounds act as flocculants and trap contaminant molecules, perhaps by means of the proportional increase in Fe(II) and Al(III) hydroxides with the number of electrodes. Likewise, the joint effect of the increase in the number of electrodes and the contact time increases the performance of the process, thus achieving a higher removal of Cr(VI) due to the electrical activity between the electrodes and the voltage supplied to the system. The contact time in the present study varied up to 30 min, because it has been reported in diverse studies that, after this time, there is a decrease in efficiency, which could be due to the effect of the EC reaching the saturation point. Therefore, unlimited growth is not achieved by increasing the reaction time (Liu et al., 2018). Moreover, as the time increases, the metal plates tend to form a loose protective film passivation layer, which influences the amount of dissolved Al(III) and Fe(II) electrode and free radicals, resulting in ions and the reduction of the number of flocculants, as well as a decrease in the oxidation effect (Das & Nandi, 2020). Another important criterion to consider in the implementation of EC is energy consumption, which is affected by the increasing time, thus implying higher processing costs (Chouhan et al., 2018).
The analysis of variance (ANOVA) is shown in Table 1, which indicates the significance of the variables in the ranges evaluated in the process of Cr(VI) removal by EC, with a p-value of less than 0,05, thus corroborating what is found in the Pareto chart in Figure 3.
Table 1. ANOVA for Cr(VI) removal efficiency
| Source | Sum of Squares | Gl | F-Ratio | P-Value |
|---|---|---|---|---|
| A: residence time | 953,266 | 1 | 157,24 | 0,0000 |
| B: voltage | 6,25 | 1 | 1,03 | 0,3397 |
| C: number of electrodes | 441,0 | 1 | 72,74 | 0,0000 |
| AB | 15,0156 | 1 | 2,48 | 0,1542 |
| AC | 435,766 | 1 | 71,88 | 0,0000 |
| BC | 22,5625 | 1 | 3,72 | 0,0898 |
| Blocks | 1,89063 | 1 | 0,31 | 0,5918 |
| Total error | 48,5 | 8 | ||
| Total (corr.) | 1 924.25 | 15 |
Source: Authors
Based on the ANOVA of the experimentally obtained data, the statistical significance of the adjusted equation was estimated by using the established variance ratio and the determination coefficients (R2). It can be inferred from the quadratic model that it was statistically significant for the efficiency of Cr(VI) removal by EC (p ≤ 0,0001). The results showed that only 3 of the 7 model terms were significant for removal efficiency: residence time (A), the number of electrodes (C), and AC interaction term (Emamjomeh et al., 2017). The R2 was higher than 90%; it showed that the variability in the adsorption could be explained by the model, with the coherence between the experimental and predicted values being significant within the process.
where RY is the percentage of removal efficiency, A is the residence time in min, B is the voltage in V, and C is the number of electrodes.
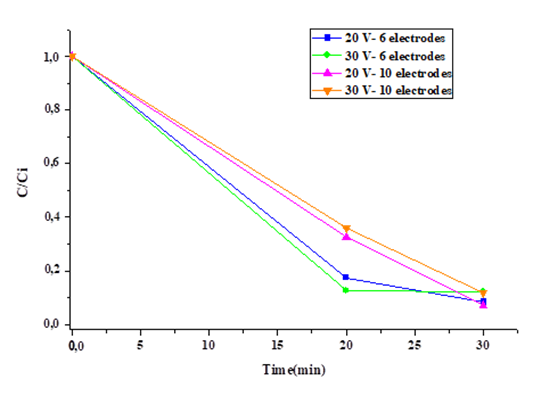
Figure 4 shows the effect of time on the C/Ci ratio of Cr(VI) for different applied voltages and the number of electrodes used at an initial concentration of 50 mg/L.
It is observed that the pH varied from an alkaline medium (10,5) to a basic medium (6,8-7). This is due to the precipitation and flotation of the chromium present in the solution, leaving water with small traces of chromium in the center of the reactor, as reported by Naghadali et al. (2019). In this regard, the speciation of chromium in aqueous solutions depends on different physical parameters such as temperature, pH, the presence of another compounds, and concentration. Nevertheless, it has been discovered that Cr(VI) can be found as HCrO4 -, CrO4 2-, or Cr2O7 2- (Martín-Domínguez et al., 2018). Thus, complex formation at the electrodes is expected because of the high state of oxidation of Chromium (Babakhouya et al., 2019). In this sense, it was demonstrated that the Cr(VI) reduction process depends on the acidity of the medium, and a greater efficiency was obtained in acidic mediums, which could be attributed to the fact that existing Cr(VI) species such as Cr2O7 2− are more easily reduced under acidic conditions than under neutral/alkaline conditions, according to Peng et al. (2019).
From Figure 4, it can be inferred that the variation in voltage does not significantly affect removal because, regardless of the voltage used, most experiments yielded removal percentages very close to and even higher than 90% (Aboulhassan et al., 2018). The best removal efficiency is given by using the configuration of 10 electrodes at 20 V for 30 min, which was due to the interaction of the number of sacrificial electrodes and the residence time, the most influential parameters in the process. This was made evident in the 30 min experiments; they obtained higher removal percentages than the 20 min treatments, which is very close to and higher than 90%. By increasing the number of electrodes and keeping the current density constant, the rate of all the reactions increases as a consequence of the increase of the anode's effective surface (Mamelkina et al., 2019). That is, by increasing the number of electrodes in the treatment, a higher concentration of aluminum in dissolution is achieved in a shorter time (Petrie et al., 2015). It is evident that, when there is a greater number of electrodes, the same removal percentage can be achieved in less time than with a smaller number of electrodes, which is why the fraction of precipitated chromium is directly proportional to the number of electrodes (Kim et al., 2020).
CONCLUSIONS
The results obtained showed efficiency in the removal of hexavalent chromium up to 92,9% using 10 electrodes and 20 V for 30 min. Thus, electrocoagulation performs better at lower voltages and longer residence times. The Cr(VI) reduction process was significantly affected by the residence time and the number of electrodes, due to the increased anode area. Electrocoagulation technology can be considered a viable alternative for the treatment of wastewater with hexavalent chromium because it allows the removal of a large amount of the contaminant in a single operation. Furthermore, chemical coagulants are not used as in conventional methods, thus making it an environmentally friendly treatment option.