Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Infectio
Print version ISSN 0123-9392
Infect. vol.17 no.4 Bogotá Oct./Dec. 2013
ORIGINAL
Increased frequency of NK and gamma delta 1 T cells in a cohort of patients with sepsis *
Mayra Diosa-Toro a, Paula Andrea Velilla a, María Teresa Rugeles a, Fabián Alberto Jaimes b,*
a Universidad de Antioquia, Grupo Inmunovirología, Sede de Investigación Universitaria, Laboratorio 532, Medellín, Colombia
b Universidad de Antioquia, Grupo Académico de Epidemiología Clínica, Hospital Universitario San Vicente de Paul; Unidad de Investigaciones, Hospital Pablo Tobón Uribe, Medellín, Colombia
Recibido el 28 de junio de 2013; aceptado el 28 de noviembre de 2013
Abstract
Introduction: The mechanisms involved in the immunopathogenesis of sepsis are not well established. The clinical and therapeutic relevance of several soluble mediators has been described and the contribution of cellular components with immunoregulatory roles has begun to be elucidated.
Objective: To describe changes in the frequency and production of IFN- γ and IL-10 occurring in NK cells and γδ T lymphocytes in a cohort of patients with different manifestations of septic syndrome.
Materials and methods: This was a prospective cohort study. Patients with sepsis (n=26), and severe sepsis (n=83) from adult emergency rooms and intensive care units, as well as healthy volunteers (n=8), were included. For all participants, the frequency and phenotype of NK cells and γδ T lymphocytes and the percentage of IFN- γ and IL-10 positive NK and γδ T cells were evaluated by flow cytometry. The NK cells were phenotyped based on the expression of CD56 and CD16 and the γδ T cells on the expression of d 1 and d 2 chains.
Results: Patients with sepsis and severe sepsis exhibited an increase in the frequency of NK cells with changes in the proportion of the CD56 bright /CD16¯, CD56 bright /CD16 dim and CD56 dim CD16¯ subpopulations; these cells showed a proinflammatory cytokine profile.
A decrease in the V d 2 subset of γδ T lymphocyte s was also observed.
Conclusions: Our results indicate a role for NK and γδ T cells during sepsis, however, their exact contribution in the pathogenesis of sepsis syndrome requires further studies.
© 2013 ACIN. Published by Elsevier España, S.L. All rights reserved.
KEYWORDS
Sepsis; NK cells; γδ T lymphocytes; Immunomodulation; IL-10; IFN- γ
Incremento en la frecuencia de células NK y células T gamma delta 1 en una cohorte de pacientes con sepsis
Resumen
Introducción: Los mecanimos involucrados en la inmunopatogénesis de las sepsis no han sido claramente establecidos. La importancia clínica y terapéutica de diferentes mediadores solubles ha sido descrita y la contribución de componentes celulares con propiedades inmunorreguladoras ha empezado a dilucidarse.
Objetivo: Describir los cambios en la frecuencia y en la producción de IFN- γ e IL-10 que se observa en células NK y linfocitos T γδ en una cohorte de pacientes con diferentes manifestaciones del síndrome séptico.
Materiales y métodos: Estudio de cohorte prospectiva, donde pacientes con sepsis (n = 26) y sepsis grave (n = 83) provenientes de las salas de emergencias y unidades de cuidado intensivo, y controles sanos (n = 8) fueron incluidos. Tanto la frecuencia y fenotipo de las células NK y T γδ como el porcentaje de células IFN- γ+ e IL-10+ fueron evaluados mediante citometría de flujo. Las células NK fueron fenotipificadas con base en la expresión de las moléculas CD56 y CD 16 y los T γδ con base en la expresión de las cadenas δ1 y δ2.
Resultados: En los pacientes con sepsis y sepsis grave se observó un incremento en la frecuencia de las células NK con cambios en las proporciones de las subpoblaciones CD56 bright /CD 16 ¯, CD56 bright /CD16 dim y CD56 dim CD16¯; en estas células se observó un perfil de citocinas proinflamarias. Se observó una reducción en el porcentaje de células Vδ2.
Conclusiones: Los resultados sugieren un papel de las células NK y linfocitos T γδ durante la sepsis; sin embargo, su contribución en la patogénesis de este síndrome requiere estudios adicionales.
© 2013 ACIN. Publicado por Elsevier España, S.L. Todos los derechos reservados.
PALABRAS CLAVE
Sepsis; Células NK; Linfocitos T γδ; Inmunomodulación; IL-10; IFN- γ
* This work was performed at Grupo Inmunovirologia, Sede de Investigación Universitaria, Calle 62 #52-59, laboratorio 532, Medellín –Colombia.
Introduction
Sepsis is considered the main cause of death in non-coronary intensive care units and one of the most common causes in the general population worldwide, with a mortality rate close to 30% and an annual increase in the incidence rate of 9%. This syndrome is complex and involves a continuum of pathological processes of varying severity, including different clinical stages such as sepsis, severe sepsis and septic shock.1 The immune response to sepsis is characterized by an initial pro-inflammatory phase manifested by high levels of the tumor necrosis factor- α (TNF- α ), interleukin (IL) 1 β , IL-6, IL-8, leukotrienes, adhesion molecules and reactive oxygen species.2 The second phase is characterized by anti-inflammatory mediators produced for limiting the inflammatory process; however, patients who survive the initial phase of sepsis may develop a state of immunosuppression.3
Inmmunosupression during sepsis, also known as compensatory anti-inflammatory response syndrome (CARS), 4 is characterized by defects in phagocytosis, antigen processing and presentation, apoptosis of dendritic cells, neutrophils and T and B lymphocytes, functional alteration of monocytes induced by the low expression of class II histocompatibility molecules, and the increased production of anti-inflammatory cytokines like IL-4, IL-10, TGF- β and the soluble receptor of TNF- α.5,6 During the immunosuppressive stage, patients are prone to acquire secondary infections and therefore the probability of death increases.7
The balance between the pro- and anti-inflammatory events of the immune response is a critical event in the outcome of the patient and, although soluble anti-inflammatory mediators have been described as key components of the evolution of sepsis, little is known about the role of lymphocytes of the innate immunity that can mediate immune regulation. Diverse evidence supports the importance of natural killer (NK) cells and γδ T lymphocytes during sepsis. 8-11 However, the role of these cells during the pathogenesis of the disease is yet controversial, mainly because most of the available knowledge comes from animal models whose data cannot be easily transferred into the humans. 8-11 Considering that innate lymphocytes could play an important role during both, the pro-inflammatory and the immune suppressive stages of sepsis, the aim of the present study was to evaluate the frequency, phenotype and functional response of NK and γδ T cells in a cohort of patients with different manifestations of the septic syndrome.
Materials and methods
Patients and study design
This cross-sectional study with prospective data collection included 114 patients with sepsis admitted to the emergency department (ED) or to intensive care units (ICUs) at the "Hospital Universitario San Vicente de Paúl" (Medellín, Colombia). This is a 550-beds, fourth level University Hospital with an admission rate of approximately 1,800 patients per month through the ED, and it is a reference institution for a region of approximately 3 million habitants. Patients in this study belong to a larger cohort examined for the research " Toward an operative diagnosis in sepsis: a latent class approach ". 12 All patients were >18 years old with clinical suspicion of infection, and were admitted in the ED or ICUs within 24 hours before recruitment to the study. Sepsis and severe sepsis were defined by clinical consensus of three experts with clinical practice in internal medicine, infectious diseases and intensive care. This consensus followed the criteria proposed by the last international conference of sepsis definitions. 13
The general protocol for each patient after recruitment and within 24 hours was: collection of baseline clinical data; calculation of entrance Sepsis Organ Failure Assessment (SOFA) score; Acute Physiology and Chronic Health Evaluation (APACHE II) score, and blood sampling (one EDTA tube of 10 ml). During seven days, patients were monitored with daily recording of any relevant data registered in medical or nurse records, using a standardized case report form. The local scientific ethical committee (" Comité de ética del Centro de Investigaciones Médicas de la Facultad de Medicina de la Universidad de Antioquia" ) approved sample collection on the basis of informed consent. To provide a panel of values from healthy donors, we included peripheral blood samples from eight adults, four males, with median age of 46 years within the range of 39-49 years.
Cell phenotyping
Peripheral blood mononuclear cells (PBMC) from patients with diagnosis of sepsis or severe sepsis were isolated using density gradients (Ficoll Histopaque 1077, Sigma Aldich) and cryopreserved until their processing. Cells were thawed after 3-5 months of freezing and in all cases viability was higher than 90%. To determine the frequency and phenotype the following combination of monoclonal antibodies were used: anti-CD3 APC-Cy7, CD16 FITC, and CD56 PE for NK cells and anti-CD3 APC-Cy7, anti- γδ TCR FITC, CD8 PE-Cy5 or anti- δ 1FITC and anti- δ 2 PE for γδ T lymphocytes. Most of the antibodies were from eBiosciencie (San Diego, CA, USA), the anti- δ 2 antibodies were from Becton Dickinson-Pharmingen (Franklin Lakes, NJ, USA) and the anti- δ 1antibody was from Thermo Scientific Pierce Biotechnology (BD Biosciences, Rockford, IL, IL USA).
To evaluate the functional profile of these cells, we determined the production of IFN- γ and IL-10 by intracellular staining, in response to phytohaemagglutinin (PHA) plus anti-CD28 antibody. Briefly, 1x10 6 PBMCs were cultured in 24-well plates coated with anti-CD28 antibody at 1 µ g/ml, and then PHA (Sigma-Aldrich, St Louis, MO, USA) was added at 6 µ g/ml. The cells were incubated at 37 ºC and 5% of CO 2 for 22 hours and 8 µ g/ml of brefeldin A (Sigma-Aldrich, St Louis, MO, USA) were added; the culture was further incubated for 26 hours under the same conditions. Next, the cells were fixed/permeabilized (staining buffer kit, e-Bioscience) and stained intracellularly using the antibodies anti-IFN- γ APC (eBioscience) and anti-IL-10 APC (BD Biosciences).
In all cases, the samples were run on a FacsCanto II flow cytometer (Becton Dickinson), collecting data at least on 5 × 10 4 lymphocytes (gated by forward and side scatter parameters) and analyzed using the Cell Quest Pro soft ware (Becton Dickinson). All appropriate isotype controls were run for each sample.
Analysis
Comparisons between groups were made with the Kruskal Wallis test and post-hoc comparisons were based on the Dunn test, considering significant differences with p values lower than 0.05. The frequency of cells subpopulations are depicted as the percentage of each cellular type among the total of peripheral lymphocytes. The results are presented as means and standard deviation or medians with minimal and maximal values, according to the type of data; in the Figures the data are represented as median and interquartile range (IQR). Functional analyses are presented as delta percentage (the percentage on stimulated cells minus and the percentage on non stimulated cells), and as ratios between the percentage of IFN- γ positive cells and the percentage of IL-10 positive cells as a way to reflect the net balance of TH1/TH2 responses, or between the percentage of δ 2 and δ 1 positive cells to demonstrate the switch in the proportion of these subpopulations in peripheral blood. For the analysis the GradPad Prims 5 software was used.
Results
Study population
A total of 114 patients were included, most of them with severe sepsis (n= 88), according to the presence of at least one organ dysfunction. However, compared to the usual presentation in sepsis, the general findings indicated a particularly young and healthy population with a median age of 50 years (37-67 years) and 53% of them (60 patients) without any previous comorbidity. Also, the average values of the SOFA (3 ± 2) and APACHE II (12 ± 5) scores suggested a relatively low severity. A microbiological confirmation of infection was possible in 44% (50 patients), blood cultures were requested in 89 patients (78%) and 27 of them (24% among the total study population) yielded a positive result. The main demographic and clinical characteristics of the patients, according to the clinical stage, are shown in Table 1.
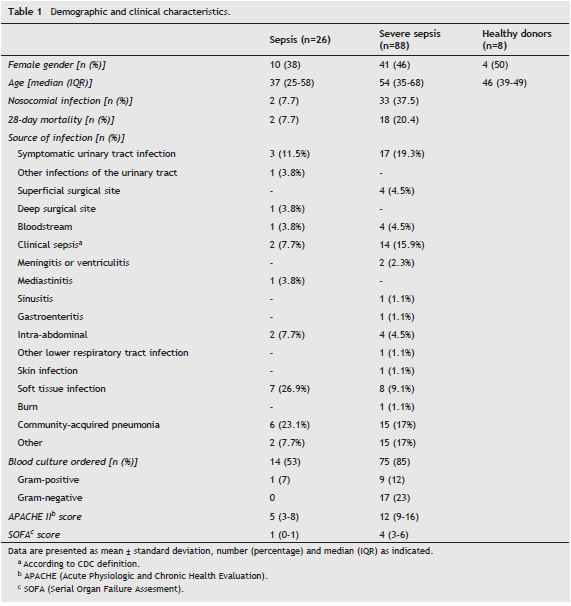
Increased frequency of NK cells during sepsis
As shown in Figure 1, the percentage of NK, assessed among the total PBMCs and defined as CD3¯ and CD56 + CD16 + / CD56 + CD16¯/CD56¯CD16 + cells was significantly increased in patients with sepsis and severe sepsis compared with healthy donors (8,26% (4-30.6) and 8.56% (0.47-61.95) vs 2.99% (1.87-10.89), p=<0.05 for both, respectively). However, no differences were observed in the percentage of these cells between groups of septic patients. In addition, no differences were found among the three groups studied regarding the frequency γδ T lymphocytes (median control group: 4.025% (0.95-6.8), patients with sepsis: 3.75% (0.64- 17,27) and patients with severe sepsis 3.76% (0.07-22.5)).
No differences were found in the absolute number of these cell populations among groups (data not shown).
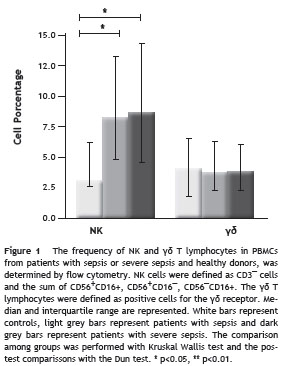
Sepsis induces changes in the proportion of NK and γδ T cells subpopulations
NK cells were divided in five subpopulations according the differential expression of CD56 and CD16 molecules. The CD56 dim CD16 + and CD56¯CD16 + subpopulations represent around 90% of NK peripheral cells, exhibit highly cytotoxic activity, are involved in the antibody-dependent cell-mediated cytotoxicity and produce low amounts of IFN- γ . The CD56 bright CD16¯, CD56 bright CD16 dim subpopulations (around 10% of peripheral NK cells) do not have high cytotoxic activity but are good producers of cytokines. Although the CD56 dim CD16¯ subpopulation is not well characterized, it has been postulated that have cytotoxic activity but also can produce cytokines. 14 In our study population, CD56 bright /CD16¯ NK cells were significantly diminished in patients with sepsis and severe sepsis compared with healthy donors (medians: 1,24% (0.06-14.31) and (3.11% (0-20.29) vs 7.11% (2.61-15.32), p<0.01 and p<0.05, respectively). The CD56 bright /CD16 dim cells were also decreased in both groups of patients compared with healthy donors but only the group of patients with severe sepsis reached statistical significance (medians: 1.70% (0-18.53), vs. 4.42% (1,2-7), p<0.05). Conversely, the CD56 dim CD16¯ subpopulation was increased in patients with severe sepsis compared with healthy donors (8.82% (0.59-49.25) vs. 4.22% (1.75-7.5), respectively, p<0.05) and also compared with patients with sepsis (4.63% (1-23.78), p<0.05). No differences were found among groups in the frequency of CD56 dim /CD16 + and CD56¯/ CD16 + NK cells (Fig. 2A).
The frequency of V δ 2 and V δ 1 T lymphocytes showed a switch in the proportion of these subsets (Fig. 2B), with a reduction in circulating V δ 2T cells and a significant increase in V δ 1 observed in both groups of patients compared with healthy donors. The median percentages of V δ 2 T cells were: 2.67% (0.55-4.7) in healthy donors vs 0.71% (0.07-14.5) and 0.69% (0-13.9) in patients with sepsis and severe sepsis p>0.05 and p<0.05, respectively).
The median percentages of V δ 1 T cells were: 0.95% (0,24-1.84) in healthy donors vs 2.37% (0.55-9.94) and 2,27% (0.07-14.18) p<0.01, for both patients with sepsis and patients with severe sepsis, respectively.
When we evaluated the δ 2/ δ 1 ratio, we found that for healthy donors it was 2.15 while for patients with sepsis and severe sepsis it was 0.31 and 0.32, respectively.
Finally, we evaluated the expression of CD8 on γδ T lymphocytes, a subpopulation related with immunoregulatory activity, but we did not find differences among groups; the CD8 negative subpopulation of the γδ T lymphocytes was the most frequent in all groups (data not shown).
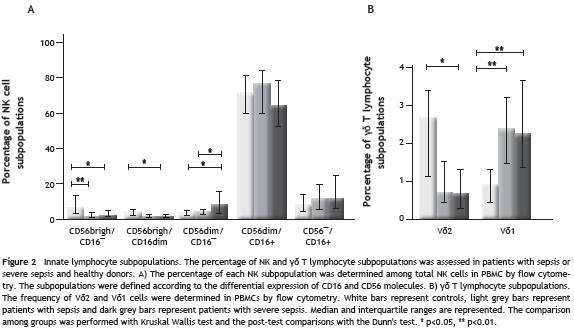
IFN- γ and IL-10 production by NK and γδ T cells
When the percentage of IFN- γ or IL-10 positive cells after stimulation with PHA/anti-CD28 was evaluated (Fig. 3), there were no significant differences in the production of IFN- γ by NK or γδ T cells among groups. However, both groups of patients have a significantly lower percentage of IL-10-positive NK cells compared with healthy donors {0.07% (-3.1%-11.45%) for patients with sepsis (0.04% (-2.54%-1.6%) and for patients with severe sepsis vs 7.12% (4.92%-16.59%) for healthy donors p<0.05 and p<0.01; respectively}. Similarly, patients with severe sepsis and sepsis, have a lower percentage of IL-10 positive γδ T cells compared with healthy donors, although the difference was only significant with severe sepsis patients {3.73% (1.16%-4,25) for healthy donors vs ¯0.17% (?7.9%-1.94%) for those with severe sepsis, p<0.01 and 0.20% (?0.66%- 3.88%), p>0.05 for sepsis group}.
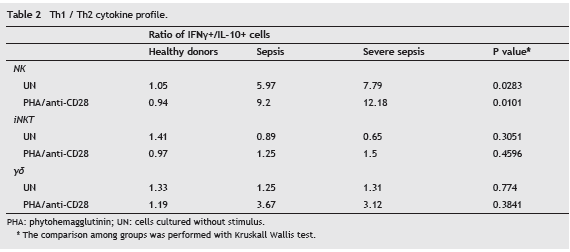
Proinflammatory cytokine profile in NK cells during sepsis
As show in Table 2, in healthy donors the ratios between IFN- γ and IL-10 expressing NK and γδ T cells were close to 1, in both unstimulated and stimulated cells. Conversely, a preferentially proinflammatory profile was observed in patients since the percentage of IFN- γ positive cells was higher than the percentage of IL-10 positive cells in NK cells (p<0.05 for both unstimulated and stimulated cells) and in γδ T cells, although in this latter cell subpopulation the difference was not significant.
Discussion
The role of innate lymphocyte subpopulations during the pathogenesis of sepsis has been recently underlined based on their immunoregulatory properties and the potential influence in the outcome of the patient. However, the characterization of these cells in patients with sepsis has not been fully established.
The role of NK cells during the pathogenesis of sepsis is controversial. 15 Here we show that the percentage of peripheral NK cells is increased in patients with sepsis, finding that is in agreement with a previous report in neonates with sepsis 16 and in patients with severe Gram-negative sepsis. 17 In addition, it was also described that patients with severe sepsis who died have higher counts of NK cells in blood and early activation patterns in comparison with those patients who survived. 18,19
Although we found that the percentage of IFN- γ -positive NK cells do not differ from that of controls, the overall pro-inflammatory response of NK cells could be higher in patients, since there was a significant decrease in the percentage of IL-10 NK positive cells when compare with controls. We speculate that the decreased percentage of IL-10 NK positive cells in patients might be related to the decrease in the CD56 bright NK subpopulations which is primarily involved in cytokine production. 20
In the context of increased percentage of NK cells and lower production of anti-inflammatory cytokines, NK cells can promote the overactive immune response observed in the initial phases of sepsis. This would be in agreement with what has been postulated in experimental models of sepsis, where there is increasing evidence of the detrimental role of NK cells during the disease. 8,10,21
Despite the low severity of our studied population we found an increase in the percentage of the subpopulation CD56 dim /CD16¯ in severe sepsis patients.
The origin and functional profile of CD56 dim /CD16¯ NK subpopulation has not yet been clarified. The increased percentage of these cells in patients with severe sepsis might suggest a possible association between a cytotoxic activity and the severity of sepsis; unfortunately, we could not perform cytotoxicity assays. In a previous study, an association between the activation of cytotoxic cells and higher mortality and severity of organ dysfunction in patients with sepsis was established. 22 However, it cannot be ensured that the increased frequency of the cytotoxic subpopulations represents an increase in cytotoxic activity, given that previous reports in other pathological contexts, such as HIV infection, have indicated that despite an increased frequency of these cells their functional activity is greatly altered. 23
γδ T cells represent a small proportion (1-10%) of the total T lymphocytes in peripheral blood, where V δ 2 T cells are the most prevalent subset (>80%). A smaller subset has the receptor V δ 1 involved in the recognition of stress-induced MHC-related antigens. 24 In contrast to previous studies, we did not find differences in the frequency of γδ T lymphocytes in PBMCs among the three groups studied. Matsushima et al. 25 reported an early decrease in the frequency of γδ T lymphocytes in whole blood from patients with SIRS and patients with sepsis, and Venet et al. 26 reported a significant decrease in γδ T lymphocytes in septic shock patients. These differences could be explained, in addition to the particular characteristics of our study population, by the type of sample, since in the studies of Venet et al. the frequency was established in peripheral blood while in our study, the frequency was determined in PBMCs.
The expression of CD8 on γδ T lymphocytes was also evaluated since the CD8 positive subpopulation has been reported to play a regulatory function in pathological conditions. 27 No differences were found among the groups. Nevertheless, the role that γδ T lymphocytes play during the disease may be attributed to other phenotypic characteristics such as the presence of the variable segments V δ 1 and V δ 2 in their receptor. In fact, there was an interesting switch with a dramatic decrease in the percentage of V δ 2 T cells and the consequent increase of V d 1 T cells in patients with sepsis. A similar change has been reported in HIV-infected individuals, particularly in patients co-infected with opportunistic pathogens. 28 The reduction of V δ 2 T cells could be related to the response to quimiotactic stimulus or to apoptosis susceptibility by sustained activation, 29,30 while the increase in peripheral V δ 1 T cells could be related to recirculation of these cells from mucosa tissues. 31
Considering the role that V δ 2 T cells play in the initial control of infections and their marked reduction in patients with sepsis, we can speculate on the importance of this subset in preventing secondary infections, which can be enhanced by the lack of effector cells, and the presence of regulatory cells that favor the expansion or acquisition of infections. However, we found a lower percentage of IL-10-positive γδ T lymphocytes in response to stimulation, a finding that suggests the importance of others immunoregulatory populations during infection. In the setting of our experimental design, it was not possible to establish whether the reduction in the V δ 2 subset of γδ T cells is a predisposing factor to the development of sepsis or is a consequence of the infection itself.
Since these entire cell subpopulations are potent producers of IFN- γ in experimental models of sepsis, they are believed to play a significant role in the dysregulated septic response. In this study we observed that both NK and γδ T cells from patients with severe sepsis tend to produce more IFN- γ than IL-10. Considering the early role of IFN- γ in promoting the functional activity of macrophages and other antigen-presenting cells, 32 and the importance of IL-10 for the regulation of the immune response, the production of these cytokines may have differential implications in the pathogenesis of the septic syndrome, depending on its progression. However, it is important to note that the ability of these cell subpopulations to produce either pro-inflammatory or anti-inflammatory cytokines are conditioned by the type of stimuli and also by the functional maturation of other cell populations, that can amplify immune responses and could be associated with deleterious or beneficial outcomes in patients with sepsis.
Taken together, the results suggest that alterations in innate immune cell subpopulations during sepsis are more evident in severe cases. These patients exhibit an increase in the frequency of NK, mainly the CD56 dim subpopulation with a proinflammatory cytokine profile and also a switch in the proportion of δ 2 and δ 1 subpopoulation. Since our study population exhibited low severity, the magnitude of the phenotypic changes in most severe cases should be much more evident. However, the clinical relevance of these findings remains to be established.
Conflicts of interest
All the authors confirm that there are no conflicts of interest associated with this publication.
Acknowledgements
We thank Dr. Anne-Lise Haenni for her suggestions to the manuscript. This study was supported by COLCIENCIAS (1115-3431-9154) and the program "Estrategia de Sostenibilidad 2013-2014 de la Universidad de Antioquia".
* This work was performed at Grupo Inmunovirologia, Sede de Investigación Universitaria, Calle 62 #52-59, laboratorio 532, Medellín –Colombia.
* Autor para correspondencia.
Correo electrónico: fjaimes@catios.udea.edu.co (F.A. Jaimes).
0123-9392/$ - see front matter © 2013 ACIN. Publicado por Elsevier España, S.L. Todos los derechos reservados.
References
1. Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63-78. [ Links ]
2. Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235-43. [ Links ]
3. van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet Infect Dis. 2008;8:32-43. [ Links ]
4. Bone RC. The sepsis syndrome. Definition and general approach to management. Clin Chest Med. 1996;17:175-81. [ Links ]
5. Wang TS, Deng JC. Molecular and cellular aspects of sepsis-induced immunosuppression. J Mol Med. 2008;86:495-506. [ Links ]
6. Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594-605. [ Links ]
7. Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, et al. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186:195-202. [ Links ]
8. Sherwood ER, Enoh VT, Murphey ED, Lin CY. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Lab Invest. 2004;84:1655-65. [ Links ]
9. Goldmann O, Chhatwal GS, Medina E. Contribution of natural killer cells to the pathogenesis of septic shock induced by Streptococcus pyogenes in mice. J Infect Dis. 2005;191:1280-6. [ Links ]
10. Enoh VT, Lin SH, Lin CY, Toliver-Kinsky T, Murphey ED, Varma TK, et al. Mice depleted of alphabeta but not gammadelta T cells are resistant to mortality caused by cecal ligation and puncture. Shock. 2007;27:507-19. [ Links ]
11. Chung CS, Watkins L, Funches A, Lomas-Neira J, Cioffi WG, Ayala A. Deficiency of gammadelta T lymphocytes contributes to mortality and immunosuppression in sepsis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1338-43. [ Links ]
12. De La Rosa GD, Valencia ML, Arango CM, Gomez CI, Garcia A, Ospina S, et al. Toward an operative diagnosis in sepsis: a latent class approach. BMC Infect Dis. 2008;8:18. [ Links ]
13. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250-6. [ Links ]
14. Penack O, Gentilini C, Fischer L, Asemissen AM, Scheibenbogen C, Thiel E, et al. CD56dimCD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia. 2005;19:835-40. [ Links ]
15. Chiche L, Forel JM, Thomas G, Farnarier C, Vely F, Blery M, et al. The role of natural killer cells in sepsis. J Biomed Biotechnol. 2011;2011:986491. [ Links ]
16. Hotoura E, Giapros V, Kostoula A, Spirou P, Andronikou S. Tracking changes of lymphocyte subsets and pre-inflammatory mediators in full-term neonates with suspected or documented infection. Scand J Immunol. 2010;73:250-5. [ Links ]
17. Giamarellos-Bourboulis EJ, Tsaganos T, Spyridaki E, Mouktaroudi M, Plachouras D, Vaki I, et al. Early changes of CD4-positive lymphocytes and NK cells in patients with severe Gram-negative sepsis. Crit Care. 2006;10:R166. [ Links ]
18. Andaluz-Ojeda D, Iglesias V, Bobillo F, Almansa R, Rico L, Gandia F, et al. Early natural killer cell counts in blood predict mortality in severe sepsis. Crit Care. 2011;15:R243. [ Links ]
19. de Pablo R, Monserrat J, Torrijos C, Martin M, Prieto A, Alvarez- Mon M. The predictive role of early activation of natural killer cells in septic shock. Crit Care. 2012;16:413. [ Links ]
20. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633-40. [ Links ]
21. Kerr AR, Kirkham LA, Kadioglu A, Andrew PW, Garside P, Thompson H, et al. Identification of a detrimental role for NK cells in pneumococcal pneumonia and sepsis in immunocompromised hosts. Microbes Infect. 2005;7:845-52. [ Links ]
22. Zeerleder S, Hack CE, Caliezi C, van Mierlo G, Eerenberg-Belmer A, Wolbink A, et al. Activated cytotoxic T cells and NK cells in severe sepsis and septic shock and their role in multiple organ dysfunction. Clin Immunol. 2005;116:158-65. [ Links ]
23. Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, et al. Characterization of CD56¯/CD16 + natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102:2886-91. [ Links ]
24. Fenoglio D, Poggi A, Catellani S, Battaglia F, Ferrera A, Setti M, et al. Vdelta1 T lymphocytes producing IFN-gamma and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood. 2009 25;113:6611-8. [ Links ]
25. Matsushima A, Ogura H, Fujita K, Koh T, Tanaka H, Sumi Y, et al. Early activation of gammadelta T lymphocytes in patients with severe systemic inflammatory response syndrome. Shock. 2004;22:11-5. [ Links ]
26. Venet F, Bohe J, Debard AL, Bienvenu J, Lepape A, Monneret G. Both percentage of gammadelta T lymphocytes and CD3 expression are reduced during septic shock. Crit Care Med. 2005;33:2836-40. [ Links ]
27. Zhou J, Appleton SE, Stadnyk A, Lee TD, Nashan BA. CD8+ gammadelta T regulatory cells mediate kidney allograft prolongation after oral exposure to alloantigen. Transpl Int. 2008;21:679-87. [ Links ]
28. De Maria A, Ferrazin A, Ferrini S, Ciccone E, Terragna A, Moretta L. Selective increase of a subset of T cell receptor gamma delta T lymphocytes in the peripheral blood of patients with human immunodeficiency virus type 1 infection. J Infect Dis. 1992;165:917-9. [ Links ]
29. Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, et al. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391-7. [ Links ]
30. Poggi A, Carosio R, Fenoglio D, Brenci S, Murdaca G, Setti M, et al. Migration of V delta 1 and V delta 2 T cells in response to CXCR3 and CXCR4 ligands in healthy donors and HIV-1-infected patients: competition by HIV-1 Tat. Blood. 2004;103:2205-13. [ Links ]
31. Poles MA, Barsoum S, Yu W, Yu J, Sun P, Daly J, et al. Human immunodeficiency virus type 1 induces persistent changes in mucosal and blood gammadelta T cells despite suppressive therapy. J Virol. 2003;77:10456-67. [ Links ]
32. Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41-101. [ Links ]













